Summary
Astract
Eprosartan is an angiotensin II receptor antagonist (angiotensin II receptor blocker [ARB]) used in the treatment of hypertension. In large, randomized trials, eprosartan (with or without hydrochlorothiazide [HCTZ]) demonstrated superior antihypertensive efficacy to that of placebo and, when administered at comparable dosage regimens, had similar blood pressure-lowering effects to enalapril. Eprosartan was generally well tolerated in clinical trials and had a lower incidence of persistent dry cough than enalapril. Eprosartan has a neutral effect on metabolic parameters, such as serum lipid levels and glucose homeostasis, and a low propensity for pharmacokinetic drug interactions. The use of eprosartan or other ARBs in combination with HCTZ tends to reverse the potassium loss associated with thiazide diuretics.
Independent of its antihypertensive effects, eprosartan was associated with improved clinical outcomes (primary composite endpoint of all causes of mortality and all cardiovascular and cerebrovascular events, including all recurrent events) compared with nitrendipine in a randomized, secondary prevention trial in hypertensive patients with previous cerebrovascular events (MOSES trial). Eprosartan also reduced blood pressure and was associated with a modest improvement in cognitive function in a large observational study in patients ≥50 years of age with newly diagnosed hypertension (OSCAR study). In both of these trials, additional antihypertensive therapy, such as HCTZ, was permitted. Therefore, eprosartan is a useful treatment option in the management of a broad range of patients with hypertension, and its use with HCTZ provides a rational combination regimen.
Pharmacological Properties
Eprosartan is a highly selective angiotensin II type 1 (AT1) receptor antagonist, with 1000-fold higher affinity for AT1 than AT2 receptors and no selectivity for adrenergic, serotonergic or other receptor types. Eprosartan has a nonbiphenyl, nontetrazole chemical structure, which is different from that of other ARBs.
Favourable effects on biomarkers of endothelial function, oxidative stress, platelet activation and haemostatic variables were demonstrated in patients with hypertension who were treated with eprosartan. Reductions in cardiac hypertrophy and mortality were among the beneficial effects of eprosartan in stroke-prone spontaneously hypertensive rats fed a high-salt, high-fat diet. In a 6-week study in patients with hypertension, eprosartan 600 mg once daily was more effective than atenolol 50 mg once daily in reducing central systolic blood pressure (SBP), aortic pulse pressure and wave reflection, but atenolol was associated with a greater effect on aortic stiffness. Eprosartan had a neutral effect on metabolic parameters, such as serum lipid levels, electrolytes and glucose homeostasis, in clinical trials.
Orally administered eprosartan has an absolute bioavailability of ≈13%, with peak plasma concentrations achieved 1–2 hours after administration. The mean terminal elimination half-life of eprosartan is ≈20 hours after multiple-dose administration. Eprosartan is primarily eliminated as unchanged drug via biliary and renal excretion. The propensity for pharmacokinetic interactions with eprosartan is low, as it is not metabolized by the cytochrome P450 (CYP) enzyme system, nor is it an inhibitor of CYP isoenzymes.
Therapeutic Efficacy
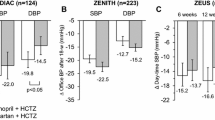
Several large (n >100), randomized, double-blind, multicentre trials showed that eprosartan 400–800 mg/day for 8 or 9 weeks achieved significantly (p≤0.01) greater reductions in sitting diastolic blood pressure (DBP) [primary endpoint] and SBP than placebo in patients with mild to moderate hypertension. Moreover, a consistent antihypertensive effect was shown throughout the once-daily dosage interval with eprosartan in a study using ambulatory blood pressure monitoring, and another trial demonstrated the superiority of eprosartan over placebo for reducing SBP (primary endpoint) in patients with isolated systolic hypertension. Active-comparator trials in patients with mild to moderate hypertension showed that comparable dosages of eprosartan and enalapril had broadly similar anti-hypertensive efficacy. A further randomized trial in patients with severe hypertension showed significantly (p<0.05) greater reductions from baseline in sitting and standing SBP (secondary endpoints) with eprosartan than with enalapril, and both agents achieved marked reductions in sitting DBP (primary endpoint) and standing DBP, with no significant between-group differences for these parameters. Concomitant HCTZ was included or permitted in some of the clinical trial protocols.
Results of the MOSES trial in hypertensive patients with previous cerebrovascular events showed that, after a mean follow-up period of 2.5 years, eprosartan-based therapy was significantly (p<0.05) more effective than nitrendipine-based therapy in reducing the primary composite endpoint of all causes of mortality and all cardiovascular and cerebrovascular events, including all recurrent events. There were no statistically significant between-group differences for changes in blood pressure. In the large, 6-month, observational OSCAR study in patients aged ≥50 years with newly diagnosed hypertension (defined as SBP ≥140mmHg), eprosartan-based therapy was associated with statistically significant (p < 0.0001) improvements for both of the co-primary endpoints (changes from baseline in SBP/DBP and Mini-Mental State Examination score), indicating not only an antihypertensive effect, but also a modest improvement in cognitive status. Results should be interpreted with caution because of inherent limitations of the observational study design.
Tolerability
Pooled data from placebo-controlled trials in patients with hypertension indicate that eprosartan has a similar tolerability profile to that of placebo. The most frequently reported adverse events with eprosartan in clinical trials were headache and gastrointestinal complaints, such as nausea, diarrhoea or vomiting. Age, race, sex, eprosartan dosage or frequency of eprosartan administration (once or twice daily) did not appear to affect the frequency of adverse events. Eprosartan was rarely associated with clinically significant laboratory abnormalities in clinical trials. Long-term (up to 2 years’) administration of eprosartan with or without HCTZ generally had a similar tolerability profile to that observed in shorter placebo-controlled trials, although there was a general trend for a higher incidence of adverse events among patients receiving combined therapy. Eprosartan was associated with a significantly (p<0.05) lower incidence of persistent dry cough than enalapril in a large, randomized, double-blind trial.





Similar content being viewed by others
References
Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure — The JNC 7 Report. JAMA 2003 May 21; 289(19): 2560–72
Wang TJ, Vasan RS. Epidemiology of uncontrolled hypertension in the United States. Circulation 2005 Sep 13; 112(11): 1651–62
Wolf-Maier K, Cooper RS, Kramer H, et al. Hypertension treatment and control in five European countries, Canada, and the United States. Hypertension 2004 Jan; 43(1): 10–7
Mancia G, De Backer G, Dominiczak A, et al. 2007 Guidelines for the management of arterial hypertension: The Task Force for the Management of Aterterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2007 Jun; 28(12): 1462–536
Lewington S, Clarke R, Qizilbash N, et al. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002 Dec 14; 360: 1903–13
Hansson L, Zanchetti A, Carruthers SG, et al. Effect of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. Lancet 1998 Jun 13; 351: 1755–62
Ogden LG, He J, Lydick E, et al. Long-term absolute benefit of lowering blood pressure in hypertensive patients according to the JNC VI risk stratification. Hypertension 2000; 35(2): 539–43
Neal B, MacMahon S, Chapman N. Effects of ACE inhibitors, calcium antagonists, and other blood-pressure-lowering drugs: results of prospectively designed overviews of randomised trials. Lancet 2000; 355: 1955–64
Givertz M. Manipulation of the renin-angiotensin system. Circulation 2001 Jul 31; 104(5): e14–8
de la Sierra A. Effects of eprosartan on target organ protection. Vasc Health Risk Manag 2006; 2(1): 79–85
Strawn WB, Ferrario CM. Mechanisms linking angiotensin II and atherogenesis. Curr Opin Lipidol 2002; 13(5): 505–12
Plosker GL, Foster RH. Eprosartan: a review of its use in the management of hypertension. Drugs 2000 Jul; 60(1): 177–201
Robins GW, Scott LJ. Eprosartan: a review of its use in the management of hypertension. Drugs 2005; 65(16): 2355–77
Teveten® (eprosartan mesylate): US prescribing information. North Chicago (IL): Abbott Laboratories, 2007 Aug
Edwards RM, Stack EJ. Angiotensin II inhibits glomerular adenylate cyclase via the angiotensin II receptor subtype 1 (AT1). J Pharmacol Exp Ther 1993; 266(2): 506–10
Edwards RM, Stack EJ, Weidley EF, et al. Characterization of renal angiotensin II receptors using subtype selective antagonists. J Pharmacol Exp Ther 1992; 260(3): 933–8
Aiyar N, Griffin E, Shu A, et al. Characterization of [3H]SKF 108566 as a radioligand for angiotensin type-1 receptor. J Recept Res 1993; 13(5): 849–61
Edwards RM, Aiyar N, Ohlstein EH, et al. Pharmacological characterization of the nonpeptide angiotensin II receptor antagonist, SKF 108566. J Pharmacol Exp Ther 1992; 260(1): 175–81
Balt JC, Mathy M-J, Pfaffendorf M, et al. Inhibition of facilitation of sympathetic neurotransmission and angiotensin II-induced pressor effects in the pithed rat: comparison between valsartan, candesartan, eprosartan and embusartan. J Hypertens 2001; 19(12): 2241–50
Nap A, Mathy MJ, Balt JC, et al. Pre- and postsynaptic inhibitory potencies of the angiotensin AT1 receptor antagonists eprosartan and candesartan. Eur J Pharmacol 2003 May 23; 469(1–3): 117–24
Ohlstein EH, Brooks DP, Feuerstein GZ, et al. Inhibition of sympathetic outflow by the angiotensin II receptor antagonist, eprosartan, but not by losartan, valsartan or irbesartan: relationship to differences in prejunctional angiotensin II receptor blockade. Pharmacology 1997; 55: 244–51
Nap A, Mathy M-J, Pfaffendorf M, et al. Different prejunctional and postjunctional responses to angiotensin II and AT1-receptor inhibition: influence of maturation. J Cardiovasc Pharmacol 2004 Mar; 43(3): 432–9
Ilson BE, Boike SC, Martin DE, et al. A dose-response study to assess the renal hemodynamic, vascular, and hormonal effects of eprosartan, an angiotensin II AT1-receptor antagonist, in sodium-replete healthy men. Clin Pharmacol Ther 1997; 63(4): 471–81
Gavras I, Gavras H. Effects of eprosartan versus enalapril in hypertensive patients on the renin-angiotensin-aldosterone system and safety parameters: results from a 26-week, double-blind, multicentre study. Eprosartan Multinational Study Group. Curr Med Res Opin 1999; 15(1): 15–24
Rahman ST, Lauten WB, Khan QA, et al. Effects of eprosartan versus hydrochlorothiazide on markers of vascular oxidation and inflammation and blood pressure (renin-angiotensin system antagonists, oxidation, and inflammation). Am J Cardiol 2002 Mar 15; 89(6): 686–90
Labíos M, Martínez M, Gabriel F, et al. Effect of eprosartan on cytoplasmic free calcium mobilization, platelet activation, and microparticle formation in hypertension. Am J Hypertens 2004 Sep; 17(9): 757–63
Leu H-B, Charng M-J, Ding P-A. A double blind randomized trial to compare the effects of eprosartan and enalapril on blood pressure, platelets, and endothelium function in patients with essential hypertension. Jpn Heart J 2004 Jul; 45(4): 623–35
Makris TK, Stavroulakis G, Papadopoulos DP, et al. Eprosartan effect on fibrinolytic/hemostatic variables in arterial hypertension: a comparative study to losartan. Drugs Exp Clin Res 2004; 30(3): 125–32
Labíos M, Martínez M, Gabriel F, et al. Effects of eprosartan on mitochondrial membrane potential and H2O2 levels in leucocytes in hypertension. J Hum Hypertens 2008 Jul; 22(7): 493–500
Labíos M, Martínez M, Gabriel F, et al. Superoxide dismutase and catalase anti-oxidant activity in leucocyte lysates from hypertensive patients: effects of eprosartan treatment. J Renin Angiotensin Aldosterone Syst 2009 Mar; 10(1): 24–30
Barone FC, Coatney RW, Chandra S, et al. Eprosartan reduces cardiac hypertrophy, protects heart and kidney, and prevents early mortality in severely hypertensive strokeprone rats. Cardiovasc Res 2001 Jun; 50(3): 525–37
Behr TM, Willette RN, Coatney RW, et al. Eprosartan improves cardiac performance, reduces cardiac hypertrophy and mortality and downregulates myocardial monocyte chemoattractant protein-1 and inflammation in hypertensive heart disease. J Hypertens 2004 Mar; 22(3): 583–92
Diamond JA, Gharavi A, Roychoudhury D, et al. Effect of long-term eprosartan versus enalapril antihypertensive therapy on left ventricular mass and coronary flow reserve in stage I-II hypertension. Curr Med Res Opin 1999; 15(1): 1–8
Cabrera Sole R, Canas J, Fernandez R, et al. Eprosartan improves left ventricular diastolic dysfunction in patients with mild to moderate hypertension: a prospective study [abstract no. P-167]. J Clin Hypertens 2006 May 1; 8 (5 Suppl. A): 76–7
Dhakam Z, McEniery CM, Yasmin, et al. Atenolol and eprosartan: differential effects on central blood pressure and aortic pulse wave velocity. Am J Hypertens 2006 Feb; 19(2): 214–9
Sock S, Perl S, Weber T, et al. Influence of eprosartan on pulse pressure, augmentation-index, pulse wave velocity and left ventricular mass index in patients with isolated systolic hypertension [abstract no. P3.190]. J Hypertens 2006 Jun 1; 24 Suppl. 4: S63
Rizos EC, Spyrou A, Liberopoulos EN, et al. Effects of eprosartan on serum metabolic parameters in patients with essential hypertension. Open Cardiovasc Med J 2007; 1: 22–6
Ilson BE, Martin DE, Boike SC, et al. The effects of eprosartan, an angiotensin II AT1 receptor antagonist, on uric acid excretion in patients with mild to moderate essential hypertension. J Clin Pharmacol 1998 May; 38(5): 437–41
Tenero D, Martin D, Ilson B, et al. Pharmacokinetics of intravenously and orally administered eprosartan in healthy males: absolute bioavailability and effect of food. Biopharm Drug Dispos 1998; 19: 351–6
Chapelsky MC, Martin DE, Tenero DM, et al. A dose proportionality study of eprosartan in healthy male volunteers. J Clin Pharmacol 1998; 38: 34–9
Tenero DM, Martin DE, Miller AK, et al. Effect of age and gender on the pharmacokinetics of eprosartan. Br J Clin Pharmacol 1998; 46: 267–70
Martin DE, Chapelsky MC, Ilson B, et al. Pharmacokinetics and protein binding of eprosartan in healthy volunteers and in patients with varying degrees of renal impairment. J Clin Pharmacol 1998; 38: 129–37
Tenero D, Martin D, Chapelsky M, et al. Effect of hepatic disease on the pharmacokinetics and plasma protein binding of eprosartan. Pharmacotherapy 1998; 18(1): 42–50
Blum RA, Kazierad DJ, Tenero DM. A review of eprosartan pharmacokinetic and pharmacodynamic drug interaction studies. Pharmacotherapy 1999; 19 (4 Pt 2): 79–85S
Teveten® HCT (eprosartan mesylate/hydrochlorothiazide): US prescribing information. North Chicago (IL): Abbott Laboratories, 2008 Oct
Meredith PA. Angiotensin II receptor antagonists alone and combined with hydrochlorothiazide: potential benefits beyond the antihypertensive effect. Am J Cardiovasc Drugs 2005; 5(3): 171–83
Patel RB, Patel UR, Rogge MC, et al. Bioavailability of hydrochlorothiazide from tablets and suspensions. J Pharm Sci 1984 Mar; 73(3): 359–61
Beermann B, Groschinsky-Grind M. Pharmacokinetics of hydrochlorothiazide in man. Eur J Clin Pharmacol 1977 Dec 2; 12(4): 297–303
Niemeyer C, Hasenfuss G, Wais U, et al. Pharmacokinetics of hydrochlorothiazide in relation to renal function. Eur J Clin Pharmacol 1983; 24(5): 661–5
White WB, Anwar YA, Mansoor GA, et al. Evaluation of the 24-hour blood pressure effects of eprosartan in patients with systemic hypertension. Am J Hypertens 2001 Dec; 14(12): 1248–55
Gradman AH, Gray J, Maggiacomo F, et al. Assessment of once-daily eprosartan, an angiotensin II antagonist, in patients with systemic hypertension. Clin Ther 1999; 21(3): 442–53
Hedner T, Himmelmann A. The efficacy and tolerance of one or two daily doses of eprosartan in essential hypertension. Eprosartan Multinational Study Group. J Hypertens 1999; 17(1): 129–36
Punzi HA, Punzi CF. Once-daily eprosartan mesylate in the treatment of elderly patients with isolated systolic hypertension: data from a 13-week double-blind, placebo-controlled, parallel, multicenter study. Eprosartan Investigation Group. J Hum Hypertens 2004 Mar 25; 18(9): 655–61
Sachse A, Verboom CN, Jäger B. Efficacy of eprosartan in combination with HCTZ in patients with essential hypertension. J Hum Hypertens 2002; 16: 169–76
Ruilope L, Jäger B, Prichard B. Eprosartan versus enalapril in elderly patients with hypertension: a double-blind, randomized trial. Blood Press 2001; 10(4): 223–9
Sega R. Efficacy and safety of eprosartan in severe hypertension. Eprosartan Multinational Study Group. Blood Press 1999; 8(2): 114–21
Elliott WJ. Double-blind comparison of eprosartan and enalapril on cough and blood pressure in unselected hypertensive patients. Eprosartan Study Group. J Hum Hypertens 1999; 13: 413–7
Derosa G, Ragonesi PD, Mugellini A, et al. Effects of telmisartan compared with eprosartan on blood pressure control, glucose metabolism and lipid profile in hypertensive, type 2 diabetic patients: a randomized, double-blind, placebo-controlled 12-month study. Hypertens Res 2004 Jul; 27(7): 457–64
Schrader J, Lüders S, Kulschewski A, et al. Morbidity and mortality after stroke, eprosartan compared with nitrendipine for secondary prevention: principal results of a prospective randomized controlled study (MOSES). Stroke 2005 Jun; 36(6): 1218–26
Hanon O, Berrou J-P, Negre-Pages L, et al. Effects of hypertension therapy based on eprosartan on systolic arterial blood pressure and cognitive function: primary results of the observational study on cognitive function and systolic blood pressure reduction open-label study. J Hypertens 2008 Aug; 26(8): 1642–50
Pathak A, Hanon O, Negre-Pages L, et al. Rationale, design and methods of the OSCAR study: observational study on cognitive function and systolic blood pressure reduction in hypertensive patients. Fundam Clin Pharmacol 2007 Apr; 21(2): 199–205
Qaseem A, Snow V, Cross Jr T, et al. Current pharmacologic treatment of dementia: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med 2008; 148(5): 370–8
Robles NR, Martin-Agueda B, Lopez-Munoz F, et al. Effectiveness of eprosartan in diabetic hypertensive patients. Eur J Intern Med 2008 Jan; 19(1): 27–31
Aranda P, Aranda FJ, Fernandez E, et al. Long-term safety and effectiveness of eprosartan in mild-to-moderate essential hypertensive patients. EPROSYST study [abstract no. P-189]. Am J Hypertens 2003 May; 16(5 Pt 2): 107A
de la Sierra A, Munoz A, Arcos E, et al. The effect of treatment with eprosartan on pulse pressure: factors predicting response. Can J Cardiol 2004 Oct; 20 Suppl. C: 17–22C
Robles NR, Martín-Águeda B, López-Munoz F, et al. Effectiveness and safety of eprosartan on pulse pressure for the treatment of hypertensive patients. Int J Clin Pract 2005 Apr; 59(4): 478–84
de la Sierra A, Munoz A, Arcos E, et al. Effect of eprosartan on pulse pressure and blood pressure components in patients with isolated systolic hypertension. Blood Press 2004 Dec; 13 Suppl. 2: 5–10
Luders S, Hammersen F, Kulschewski A, et al. Stress-associated hypertension at the work place: results of the STARLET project [in German]. Dtsch Med Wochenschr 2006 Nov 17; 131(46): 2580–5
Levine B. Eprosartan provides safe and effective long-term maintenance of blood pressure control in patients with mild to moderate essential hypertension. Curr Med Res Opin 2001; 17(1): 8–17
de la Sierra A. Effects of eprosartan on pulse pressure. J Clin Basic Cardiol 2005; 8: 11–4
Eprosartan: master summary of product characteristics (SmPC). Chatillon-sur-Chalaronne, France: Solvay Pharmaceuticals, 2008 Jul 31
Gavras I, Gavras H. Safety and tolerability of eprosartan. Pharmacotherapy 1999; 19 (4 Pt 2 Suppl.): 102–7S
Böhm M, Sachse A. Safety and tolerability of eprosartan in combination with hydrochlorothiazide. Drug Saf 2002; 25(8): 599–611
Schwander B, Gradl B, Zollner Y, et al. Cost-utility analysis of eprosartan compared to enalapril in primary prevention and nitrendipine in secondary prevention in Europe — the HEALTH model. Value Health 2009 Mar 10; 12(6): 857–71
Cooper WO, Hernandez-Diaz S, Arbogast PG, et al. Major congenital malformations after first-trimester exposure to ACE inhibitors. N Engl J Med 2006 Jun 8; 354(23): 2443–51
Sealey JE, Itskovitz-Eldor J. ACE inhibitors and major congenital malformations [letter]. N Engl J Med 2006 Sep 21; 355(12): 1280–1
ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA 2002 Dec 18; 288(23): 2981–97
Dahlöf B, Devereux RB, Kjeldsen SE, et al. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet 2002 Mar 23; 359(9311): 995–1003
Weber MA, Julius S, Kjeldsen SE, et al. Blood pressure dependent and independent effects of antihypertensive treatment on clinical events in the VALUE trial. Lancet 2004 Jun 19; 363(9426): 2049–51
Blankestijn PJ, Rupp H. Clinical profile of eprosartan: a different angiotensin II receptor blocker. Cardiovasc Hematol Agents Med Chem 2008 Oct; 6(4): 253–7
Larsen K, Hornnes N, Boysen G. Does the MOSES study provide sufficient evidence for eprosartan against nitrendipine? [letter]. Stroke 2006 Jun; 37(6): 1357; author reply 1358
Boulanger JM, Hill MD. Morbidity and mortality after stroke — eprosartan compared with nitrendipine for secondary prevention: principal results of a prospective randomized controlled study (MOSES) [letter]. Stroke 2006 Feb; 37(2): 335–6; author reply 338
Owen A. MOSES: superiority or noninferiority? [letter]. Stroke 2006 Feb; 37(2): 337–8; author reply 338
Fournier A, Choukroun G, Modeliar SS, et al. Does the MOSES trial establish superiority of AT1-receptor blockers over dihydropyridine/calcium antagonists in secondary stroke prevention? [letter]. Stroke 2006 Feb; 37(2): 336–7; author reply 338
Diener H-C, Sacco RL, Yusuf S, et al. Effects of aspirin plus extended-release dipyridamole versus clopidogrel and telmisartan on disability and cognitive function after recurrent stroke in patients with ischaemic stroke in the Prevention Regimen for Effectively Avoiding Second Strokes (PRoFESS) trial: a double-blind, active and placebo-controlled study. Lancet Neurol 2008 Oct; 7: 875–84
Smith DHG. Comparison of angiotensin II type 1 receptor antagonists in the treatment of essential hypertension. Drugs 2008; 68(9): 1207–25
DeBacker G. The SCORE model in the POWER study: an attempt to focus the limited resources for prevention on patients with greatest need. Curr Med Res Opin 2007 Nov; 23 Suppl. 5: S19–24
Author information
Authors and Affiliations
Corresponding author
Additional information
Various sections of the manuscript reviewed by: E. Agabiti-Rosei, Department of Medical and Surgical Sciences, Clinica Medica, University of Brescia, Brescia, Italy; A. de la Sierra, Hypertension Unit, Department of Internal Medicine, Hospital Clinic, University of Barcelona, Barcelona, Spain; J. Minami, Teshima Clinic, Kure, Hiroshima, Japan; H.A. Punzi, Trinity Hypertension Research Institute, Punzi Medical Center, Carrollton, Texas, USA; N.R. Robles, Department of Nephrology, Hospital Infanta Cristina, Badajoz, Spain.
Data Selection
Sources: Medical literature published in any language since 1980 on ‘eprosartan’, identified using MEDLINE and EMBASE, supplemented by AdisBase (a proprietary database). Additional references were identified from the reference lists of published articles. Bibliographical information, including contributory unpublished data, was also requested from the company developing the drug.
Search strategy: MEDLINE, EMBASE and AdisBase search terms were ‘eprosartan’ or ‘eprosartan/hydrochlorothiazide’ or ‘eprosartan combined with hydrochlorothiazide’. Searches were last updated 23 October 2009.
Selection: Studies in patients with hypertension who received eprosartan. Inclusion of studies was based mainly on the methods section of the trials. When available, large, well controlled trials with appropriate statistical methodology were preferred. Relevant pharmacodynamic and pharmacokinetic data are also included.
Index terms: Eprosartan, hypertension, pharmacodynamics, pharmacokinetics, therapeutic use, tolerability.
Rights and permissions
About this article
Cite this article
Plosker, G.L. Eprosartan. Drugs 69, 2477–2499 (2009). https://doi.org/10.2165/11203980-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11203980-000000000-00000