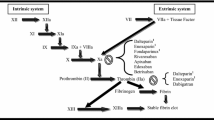
Abstract
Tinzaparin sodium (Innohep®) is a low molecular weight heparin (LMWH) that is effective in the prevention and treatment of deep vein thrombosis (DVT) and/or pulmonary embolism (PE), and in maintaining the patency of haemodialysis circuits in adult patients. In terms of preventing DVT and/or PE, therapy with subcutaneous tinzaparin sodium was more effective than oral warfarin and equivalent to subcutaneous enoxaparin sodium in patients undergoing orthopaedic surgery, and did not significantly differ from that of subcutaneous unfractionated heparin (UFH) in patients undergoing general surgery. In the initial therapy of adult patients with DVT and/or PE, subcutaneous tinzaparin sodium was at least as effective as intravenous UFH and did not significantly differ from subcutaneous dalteparin sodium. Various other studies have demonstrated that the long-term efficacy of subcutaneous tinzaparin sodium in the treatment of patients with DVT and/or PE was sustained for a total period of up to 12 months. Tinzaparin sodium was also demonstrated to be effective in maintaining the patency of haemodialysis circuits in adult patients with end-stage renal failure. In clinical studies, tinzaparin sodium was generally well tolerated in the prevention and treatment of DVT and/or PE in adult patients, including in elderly patients, and in patients undergoing haemodialysis. As expected, bleeding complications were the most frequently occurring adverse event. Thus, available data indicate that tinzaparin sodium is a useful option in the prevention and treatment of DVT and/or PE, and in maintaining the patency of haemodialysis circuits in adult patients.








Similar content being viewed by others
References
Alexander JH, Singh KP. Inhibition of factor Xa: a potential target for the development of new anticoagulants. Am J Cardiovasc Drugs 2005; 5(5): 279–90
Tincani E, Crowther MA, Turrini F, et al. Prevention and treatment of venous thromboembolism in the elderly patient. Clin Interv Aging 2007; 2(2): 237–46
Nutescu EA, Shapiro NL, Feinstein H, et al. Tinzaparin: considerations for use in clinical practice. Ann Pharmacother 2003 Dec; 37(12): 1831–40
Simonneau G, Sors H, Charbonnier B, et al. A comparison of low-molecular-weight heparin with unfractionated heparin for acute pulmonary embolism. N Engl J Med 1997; 337(10): 663–9
Clark NP. Low-molecular-weight heparin use in the obese, elderly, and in renal insufficiency. Thromb Res 2008; 123 Suppl. 1: S58–61
Hull RD, Pineo GF, Brant RF, et al. Long-term lowmolecular-weight heparin versus usual care in proximalvein thrombosis patients with cancer. Am J Med 2006 Dec; 119(12): 1062–72
Lim W, Dentali F, Eikelboom JW, et al. Meta-analysis: lowmolecular-weight heparin and bleeding in patients with severe renal insufficiency. Ann Intern Med 2006 May 2; 144(9): 673–84
U.S. Pharmacopeia. 2009 USP dictionary of USAN and international drug names. Rockville (MD): U.S. Pharmacopeia, 2009
Nader HB, Walenga JM, Berkowitz SD, et al. Preclinical differentiation of low molecular weight heparins. Semin Thromb Hemost 1999; 25 Suppl. 3: 63–72
Linhardt RJ, Gunay NS. Production and chemical processing of low molecular weight heparins. Semin Thromb Hemost 1999; 25 Suppl. 3: 5–16
Cheer SM, Dunn CJ, Foster R. Tinzaparin sodium: a review of its pharmacology and clinical use in the prophylaxis and treatment of thromboembolic disease. Drugs 2004; 64(13): 1479–502
Friedel HA, Balfour JA. Tinzaparin: a review of its pharmacology and clinical potential in the prevention and treatment of thromboembolic disorders. Drugs 1994; 48(4): 638–60
Sweetman SC, editor. Martindale: the complete drug reference. 36th ed. London: Pharmaceutical Press, 2009
Kuczka K, Baum K, Picard-Willems B, et al. Long-term administration of LMWH: pharmacodynamic parameters under therapeutic or prophylactic regimen of enoxaparin or tinzaparin in neurological rehabilitation patients. Thrombosis Res 2009 Nov; 124(5): 625–30
Bara L, Planes A, Samama M-M. Occurrence of thrombosis and haemorrhage, relationship with anti-Xa, anti-IIa activities, and D-dimer plasma levels in patients receiving a low molecular weight heparin, enoxaparin or tinzaparin, to prevent deep vein thrombosis after hip surgery. Br J Haematol 1999; 104: 230–40
Christidou FN, Frangia TK, Bamichas GI, et al. Comparison of two low-molecular weight heparins (LMWHs), tinzaparin and bemiparin, during hemodialysis. Int J Clin Pharmacol Ther 2005 Jul; 43(7): 335–8
Sabry A, Taha M, Nada M, et al. Anticoagulation therapy during haemodialysis: a comparative study between two heparin regimens. Blood Coagul Fibrinolysis 2009 Jan; 20(1): 57–62
Badawi L, Akeel N, Shaheen FAM, et al. Dose and lipid lowering effect of tinzaparin sodium: a single center experience. Saudi J Kidney Dis Transpl 2005 Apr–Jun; 16(2): 161–5
Koutsikos D, Fourtounas C, Kapetanaki A, et al. A crossover study of a new low molecular weight heparin (Logiparin®) in hemodialysis. Int J Artif Organs 1996; 19(8): 467–71
Gouin-Thibault I, Pautas E, Depasse F, et al. Heparinreleasable TFPI is not depleted after repeated injections of tinzaparin at therapeutic dosages for up to 30 days. J Thromb Haemost 2003; 1: 2694–5
Elisaf MS, Germanos NP, Bairaktari HT, et al. Effects of conventional vs. low-molecular-weight heparin on lipid profile in hemodialysis patients. Am J Nephrol 1997; 17: 153–7
Akiba T, Tachibana K, Ozawa K, et al. Long-term use of low molecular weight heparin ameliorates hyperlipidemia in patients on hemodialysis. ASAIO J 1992; 38(3): M326–30
Fossler MJ, Barrett JS, Hainer JW, et al. Pharmacodynamics of intravenous and subcutaneous tinzaparin and heparin in healthy volunteers. Am J Health Syst Pharm 2001 Sep 1; 58(17): 1614–21
Mätzsch T, Bergqvist D, Hedner U, et al. Effects of an enzymatically depolymerized heparin as compared with conventional heparin in healthy volunteers. Thromb Haemost 1987; 57(1): 97–101
Holst J, Lindblad B, Bergqvist D, et al. Protamine neutralization of intravenous and subcutaneous low-molecularweight heparin (tinzaparin, Logiparin™): an experimental investigation in healthy volunteers. Blood Coagul Fibrinolysis 1994; 5(5): 795–803
Mousa SA, Bozarth J, Barrett JS. Pharmacodynamic properties of the low molecular weight heparin, tinzaparin: effect of molecular weight distribution on plasma tissue factor pathway inhibitor in healthy human subjects. J Clin Pharmacol 2003 Jul; 43(7): 727–34
Depasse F, González de Suso MJ, Lagoutte I, et al. Comparative study of the pharmacokinetic profiles of two LMWHs — bemiparin (3500 IU, anti-Xa) and tinzaparin (4500 IU, anti-Xa) — administered subcutaneously to healthy male volunteers. Thromb Res 2003 Jan 25; 109(2–3): 109–17
Ellison J, Thomson AJ, Conkie JA, et al. Thromboprophylaxis following caesarean section: a comparison of the antithrombotic properties of three low molecular weight heparins — dalteparin, enoxaparin and tinzaparin. Thromb Haemost 2001 Dec; 86(6): 1374–8
Yoneda M, Brosnan JF, Norris LA, et al. The effect of LMWH (tinzaparin) on coagulation and fibrinolytic activation in pregnant women at risk of thrombosis. Thromb Res 2006; 117(3): 283–90
Crowther MA, Berry LR, Monagle PT, et al. Mechanisms responsible for the failure of protamine to inactivate lowmolecular-weight heparin. Br J Haematol 2002; 116(1): 178–86
Sobel M, Adelman B. Characterization of platelet binding of heparins and other glycosaminoglycans. Thromb Res 1988; 50(6): 815–26
Young E, Wells P, Holloway S, et al. Ex-vivo and in-vitro evidence that low molecular weight heparins exhibit less binding to plasma proteins than unfractionated heparin. Thromb Haemost 1994; 71(3): 300–4
Padilla A, Gray E, Pepper DS, et al. Inhibition of thrombin generation by heparin and low molecular weight (LMW) heparins in the absence and presence of platelet factor 4 (PF4). Br J Haematol 1992 Oct; 82: 406–13
Gerotziafas GT, Petropoulou AD, Verdy E, et al. Effect of the anti-factor Xa and anti-factor IIa activities of lowmolecular-weight heparins upon the phases of thrombin generation. J Thromb Haemost 2007 May; 5(5): 955–62
Leo Laboratories Limited. Innohep 10,000 IU/ml and Innohep syringe 10,000 IU/ml: summary of product characteristics [online]. Available from URL: http://emc.medicines.org.uk/ [Accessed 2010 Apr 12]
Leo Laboratories Limited. Innohep 20,000 IU/ml and Innohep syringe 20,000 IU/ml: summary of product characteristics [online]. Available from URL: http://emc.medcines.org.uk/ [Accessed 2010 Apr 12]
Leo Pharmaceutical Products. Innohep®: tinzaparin sodium injection [online]. Available from URL: http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/020484s013lbl.pdf [Accessed 2010 Apr 12]
Weitz JI. Low-molecular-weight heparins [published erratum appears in N Engl J Med 1997 Nov 20; 337 (21): 1567]. N Engl J Med 1997 Sep 4; 337(10): 688–98
Planès A, Samama MM, Lensing AWA, et al. Prevention of deep vein thrombosis after hip replacement: comparison between two low-molecular-weight heparins, tinzaparin and enoxaparin. Thromb Haemost 1999; 81: 22–5
Cambus J-P, Saivin S, Heilmann J-J, et al. The pharmacodynamics of tinzaparin in healthy volunteers. Br J Haematol 2002 Mar; 116(3): 649–52
Barrett JS, Gibiansky E, Hull RD, et al. Population pharmacodynamics in patients receiving tinzaparin for the prevention and treatment of deep vein thrombosis. Int J Clin Pharmacol Ther 2001 Oct; 39(10): 431–46
Johansen PB, Rasmussen SN, Østergaard PB. Pharmacokinetics of tinzaparin (Logiparin®) — a low molecular weight heparin — after single and repeated intravenous administration in rats. Thromb Res 1994 Aug 15; 75(4): 453–64
Mahé I, Aghassarian M, Drouet L, et al. Tinzaparin and enoxaparin given at prophylactic dose for eight days in medical elderly patients with impaired renal function: a comparative pharmacokinetic study. Thromb Haemost 2007 Apr; 97(4): 581–6
Siguret V, Pautas E, Février M, et al. Elderly patients treated with tinzaparin (Innohep®) administered once daily (175 anti-Xa IU/kg): anti-Xa and anti-IIa activities over 10 days. Thromb Haemost 2000 Nov; 84(5): 800–4
Siguret V, Leizorovicz A, Pautas E, et al. No accumulation of peak anti-Xa activity of tinzaparin in elderly patients with moderate to severe renal impairment: a substudy of IRIS clinical trial [abstract no. 172]. Blood 2009; 114(22): 77
Pautas E, Gouin I, Bellot O, et al. Safety profile of tinzaparin administered once daily at a standard curative dose in two hundred very elderly patients. Drug Saf 2002; 25(10): 725–33
Kuhle S, Massicotte P, Dinyari M, et al. Dose-finding and pharmacokinetics of therapeutic doses of tinzaparin in pediatric patients with thromboembolic events. Thromb Haemost 2005 Dec; 94(6): 1164–71
Hainer JW, Barrett JS, Assaid CA, et al. Dosing in heavyweight/obese patients with the LMWH, tinzaparin: a pharmacodynamic study. Thromb Haemost 2002 May; 87(5): 817–23
Hull RD, Raskob GE, Pineo GF, et al. Subcutaneous lowmolecular-weight heparin compared with continuous intravenous heparin in the treatment of proximal-vein thrombosis. N Engl J Med 1992 Apr 9; 326(15): 975–82
Smith MP, Norris LA, Steer PJ, et al. Tinzaparin sodium for thrombosis treatment and prevention during pregnancy. Am J Obstet Gynecol 2004; 190(2): 495–501
Parent F, Deruelle P, Jaïs X, et al. Treatment of venous thromboembolism during pregnancy with a once-daily regimen of LMWH [abstract no. PP-MO-376]. J Thromb Haemost 2009 Jul; 7 Suppl. 2: 445
Andersen AS, Berthelsen JG, Bergholt T. Venous thromboembolism in pregnancy: prophylaxis and treatment with low molecular weight heparin. Acta Obstet Gynecol Scand 2010; 89(1): 15–21
Ní Áinle F, Wong A, Appleby N, et al. Efficacy and safety of once daily low molecular weight heparin (tinzaparin sodium) in high risk pregnancy. Blood Coagul Fibrinolysis 2008 Oct; 19(7): 689–92
Hull R, Raskob G, Pineo G, et al. A comparison of subcutaneous low-molecular-weight heparin with warfarin sodium for prophylaxis against deep-vein thrombosis after hip or knee implantation. N Engl J Med 1993 Nov 4; 329(19): 1370–6
Lassen MR, Borris LC, Christiansen HM, et al. Prevention of thromboembolism in 190 hip arthroplasties: comparison of LMW heparin and placebo. Acta Orthop Scand 1991 Feb; 62(1): 33–8
Leizorovicz A, Picolet H, Peyrieux JC, et al. Prevention of perioperative deep vein thrombosis in general surgery: a multicentre double blind study comparing two doses of Logiparin and standard heparin. Br J Surg 1991; 78(4): 412–6
Bergqvist D, Flordal PA, Friberg B, et al. Thromboprophylaxis with a low molecular weight heparin (tinzaparin) in emergency abdominal surgery: a double-blind multicenter trial. Vasa 1996; 25(2): 156–60
Jørgensen PS, Warming T, Hansen K, et al. Low molecular weight heparin (Innohep) as thromboprophylaxis in outpatients with a plaster cast: a venografic controlled study. Thromb Res 2002 Mar 15; 105(6): 477–80
Lassen MR, Borris LC, Jensen HP, et al. Dose relation in the prevention of proximal vein thrombosis with a low molecular weight heparin (tinzaparin) in elective hip arthroplasty. Clin Appl Thromb Hemost 2000 Jan; 6(1): 53–7
Lausen I, Jensen R, Jorgensen LN, et al. Incidence and prevention of deep venous thrombosis occurring late after general surgery: randomised controlled study of prolonged thromboprophylaxis. Eur J Surg 1998; 164(9): 657–63
Green D, Lee MY, Lim AC, et al. Prevention of thromboembolism after spinal cord injury using low-molecularweight heparin. Ann Intern Med 1990 Oct 15; 113: 571–4
Hauch O, Jørgensen LN, Kølle TR, et al. Low molecular weight heparin (Logiparin™) as thromboprophylaxis in elective abdominal surgery: a dose finding study. Acta Chir Scand Suppl 1988; 543: 90–5
Green D, Chen D, Chmiel JS, et al. Prevention of thromboembolism in spinal cord injury: role of low molecular weight heparin. Arch Phys Med Rehabil 1994; 75: 290–2
Planès A. An equivalence study of two low-molecularweight heparins in the prevention and treatment of deepvein thrombosis after total hip replacement. Semin Thromb Hemost 2000; 26 Suppl. 1: 57–60
Wells PS, Anderson DR, Rodger MA, et al. A randomized trial comparing 2 low-molecular-weight heparins for the outpatient treatment of deep vein thrombosis and pulmonary embolism. Arch Intern Med 2005 Apr 11; 165(7): 733–8
Hull RD, Raskob GE, Brant RF, et al. Low-molecularweight heparin vs heparin in the treatment of patients with pulmonary embolism. Arch Intern Med 2000 Jan 24; 160(2): 229–36
Hull RD, Pineo GF, Brant RF, et al. Self-managed longterm low-molecular-weight heparin therapy: the balance of benefits and harms. Am J Med 2007 Jan; 120(1): 72–82
Hull RD, Pineo GF, Brant R, et al. Home therapy of venous thrombosis with long-term LMWH versus usual care: patient satisfaction and post-thrombotic syndrome. Am J Med 2009 Aug; 122(8): 762–9.e3
Romera A, Cairols MA, Vila-Coll R, et al. A randomised open-label trial comparing long-term sub-cutaneous lowmolecular-weight heparin compared with oral-anticoagulant therapy in the treatment of deep venous thrombosis. Eur J Vasc Endovasc Surg 2009 Mar; 37(3): 349–56
Lapidus L, Börretzen J, Fahlén M, et al. Home treatment of deep vein thrombosis: an out-patient treatment model with once-daily injection of low-molecular-weight heparin (tinzaparin) in 555 patients. Pathophysiol Haemost Thromb 2002 Mar; 32(2): 59–66
Leizorovicz A, on behalf of the IRIS Group. Tinzaparin compared to unfractionated heparin for initial treatment of deep vein thrombosis in very elderly patients with renal insufficiency: the IRIS trial [abstract no. 434]. Blood 2008 Nov 16; 112(11): 166
Beijering RJR, ten Cate H, Stevens P, et al. Randomised long-term comparison of tinzaparin and dalteparin in haemodialysis. Clin Drug Investig 2003; 23(2): 85–97
Simpson HKL, Baird J, Allison M, et al. Long-term use of the low molecular weight heparin tinzaparin in haemodialysis. Haemostasis 1996; 26: 90–7
Ryan KE, Lane DA, Flynn A, et al. Dose finding study of low molecular weight heparin, Innohep, in haemodialysis. Thromb Haemost 1991; 66(3): 277–82
Bramham K, Varrier M, Asgari E, et al. Comparison of tinzaparin and unfractionated heparin as anticoagulation on haemodialysis: equal safety, efficacy and economical parity. Nephron Clin Pract 2008; 110(2): c107–13
Egfjord M, Rosenlund L, Hedegaard B, et al. Dose titration study of tinzaparin, a low molecular weight heparin, in patients on chronic hemodialysis. Artif Organs 1998; 22(8): 633–7
Lord H, Jean N, Dumont M, et al. Comparison between tinzaparin and standard heparin for chronic hemodialysis in a Canadian center. Am J Nephrol 2002; 22(1): 58–66
Leo Pharma Inc. Product monograph PRinnohep® (tinzaparin sodium injection) anticoagulant/antithrombotic agent [online]. Available from URL: http://www.hc-sc.gc.ca [Accessed 2010 Mar 15]
Bates SM, Ginsberg JS. Clinical practice: treatment of deepvein thrombosis. N Engl J Med 2004 Jul 15; 351(3): 268–77
Torbicki A, Perrier A, Konstantinides S, et al. Guidelines on the diagnosis and management of acute pulmonary embolism: the Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). Eur Heart J 2008 Sep; 29(18): 2276–315
Krishnan JA, Segal JB, Streiff MB, et al. Treatment of venous thromboembolism with low-molecular-weight heparin: a synthesis of the evidence published in systematic literature reviews. Respir Med 2004 May; 98(5): 376–86
Segal JB, Streiff MB, Hofmann LV, et al. Management of venous thromboembolism: a systematic review for a practice guideline. Ann Intern Med 2007 Feb 6; 146(3): 211–22
Myers J. Selecting an agent for prophylaxis of venous thromboembolism. Am J Health Syst Pharm 2006 Dec 15; 63(24): 2448–50
Messmore HL, Coyne E, Wehrmacher WH, et al. Studies comparing low molecular weight heparin with heparin for the treatment of thromboembolism: a literature review. Curr Pharm Des 2004; 10(9): 1001–10
Wolf H. Low-molecular-weight heparin. Med Clin North Am 1994 May; 78(3): 733–43
Kearon C, Kahn SR, Agnelli G, et al. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th edition). Chest 2008 Jun; 133 (6 Suppl.): 454S–545S
Geerts WH, Bergqvist D, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th edition). Chest 2008 Jun; 133 (6 Suppl.): 381S–453S
Patel JP, Hunt BJ. Where do we go now with low molecular weight heparin use in obstetric care? J Thromb Haemost 2008 Sep; 6(9): 1461–7
Lykke JA, Grønlykke T, Langhoff-Roos J. Treatment of deep venous thrombosis in pregnant women. Acta Obstet Gynecol Scand 2008; 87(11): 1248–51
Bates SM, Greer IA, Pabinger I, et al. Venous thromboembolism, thrombophilia, antithrombotic therapy, and pregnancy: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th edition). Chest 2008 Jun; 133 (6 Suppl.):844S–86S
Spyropoulos AC, Merli G. Management of venous thromboembolism in the elderly. Drugs Aging 2006; 23(8): 651–71
Author information
Authors and Affiliations
Corresponding author
Additional information
Various sections of the manuscript reviewed by: D. Bergqvist, Department of Surgery, University Hospital, Uppsala, Sweden; R.D. Hull, Thrombosis Research Unit, Foothills Hospital, University of Calgary, Calgary, Alberta, Canada; M.R. Lassen, Department of Orthopaedics, Spine Clinic, Clinical Trial Unit, Hørsholm Hospital, University of Copenhagen, Hørsholm, Denmark; A. Planès, Clinique Chirurgicale du Mail, La Rochelle, France; V. Siguret, Laboratoire d’Hématologie, Assistance-Publique Hôpitaux de Paris, Hôpital Charles Foix, Ivry-sur-Seine, France.
Data Selection
Sources: Medical literature published in any language since 1980 on ‘tinzaparin’, identified using MEDLINE and EMBASE, supplemented by AdisBase (a proprietary database). Additional references were identified from the reference lists of published articles. Bibliographical information, including contributory unpublished data, was also requested from the company developing the drug.
Search strategy: MEDLINE, EMBASE and AdisBase search terms were ([‘tinzaparin’ or ‘tinzaparin sodium’] and [‘deep vein thrombosis’ or ‘venous thrombosis’ or ‘venous thromboembolism’ or ‘pulmonary embolism’]). Searches were last updated on 14 June 2010.
Selection: Studies in the prevention and treatment of deep vein thrombosis, including pulmonary embolism, and in maintaining the patency of haemodialysis circuits in patients who received tinzaparin sodium. Inclusion of studies was based mainly on the methods section of the trials. When available, large, well controlled trials with appropriate statistical methodology were preferred. Relevant pharmacodynamic and pharmacokinetic data are also included.
Index terms: Tinzaparin sodium, deep vein thrombosis, haemodialysis, pulmonary embolism, pharmacodynamics, pharmacokinetics, therapeutic use, tolerability.
Rights and permissions
About this article
Cite this article
Hoy, S.M., Scott, L.J. & Plosker, G.L. Tinzaparin Sodium. Drugs 70, 1319–1347 (2010). https://doi.org/10.2165/11203710-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11203710-000000000-00000