Summary
Abstract
Peginterferon-α-2a (40 kD) [Pegasys®] comprises an inert, branched, 40 kD polyethylene glycol (PEG) moiety attached to interferon-α-2a. Subcutaneous peginterferon-α-2a (40 kD) is indicated for the treatment of adults with hepatitis B e antigen (HBeAg)-positive or -negative chronic hepatitis B who have compensated liver disease with evidence of viral replication and hepatic inflammation.
Subcutaneous peginterferon-α-2a (40 kD) has antiviral and immunomodulatory properties and a convenient once-weekly administration schedule. Forty-eight weeks of therapy with peginterferon-α-2a (40 kD) with or without lamivudine was more effective than lamivudine alone in achieving a sustained response in patients with HBeAg-positive or -negative chronic hepatitis B. A long-term follow-up study in patients with HBeAg-positive disease who received peginterferon-α-2a (40 kD) monotherapy revealed an HBeAg seroconversion rate of 42%, 1 year after the end of treatment. A long-term follow-up study in patients with HBeAg-negative disease who received peginterferon-α-2a (40 kD) with or without lamivudine revealed hepatitis B surface antigen (HBsAg) clearance in 12% of patients and inactive chronic hepatitis B in 17% of patients, 5 years after the end of treatment. Various predictors of response may be useful in terms of identifying patients who may be candidates for shorter or longer peginterferon-α-2a (40 kD) treatment durations. For example, quantifying serum HBeAg (in HBeAg-positive disease) and HBsAg levels during therapy may be useful. Adverse events typical of the influenza-like symptoms seen with α-interferons occurred more frequently in patients with chronic hepatitis B receiving peginterferon-α-2a (40 kD) with or without lamivudine than in those receiving lamivudine alone. In conclusion, peginterferon-α-2a (40 kD) is a valuable option for the first-line treatment of HBeAg-negative or -positive chronic hepatitis B.
Pharmacological Properties
Peginterferon-α-2a (40 kD) has antiviral and immunomodulatory effects. In patients with HBeAg-negative chronic hepatitis B, hepatitis B virus (HBV) kinetics in peginterferon-α-2a (40 kD) recipients differed to those in lamivudine recipients, most likely reflecting different mechanisms of action. During the first month of therapy, viral load decay showed a biphasic pattern with peginterferon-α-2a (40 kD) alone and a multiphasic pattern with peginterferon-α-2a (40 kD) plus lamivudine or lamivudine alone. Although the first slope of viral load decay, reflecting a direct antiviral effect, was significantly slower with peginterferon-α-2a (40 kD) than with lamivudine, peginterferon-α-2a (40 kD) may clear infected cells to a greater extent than lamivudine, possibly reflecting its immunomodulatory activity.
Attaching the large branched 40 kD polyethylene glycol molecule to interferon-α-2a optimizes its pharmacokinetics, allowing for once-weekly administration. Serum concentrations of peginterferon-α-2a (40 kD) are sustained over a 1-week period, and steady-state serum concentrations were reached within 5-8 weeks with once-weekly administration of peginterferon-α-2a (40 kD).
Therapeutic Efficacy
Forty-eight weeks therapy with peginterferon-α-2a (40 kD) with or without lamivudine was more effective than lamivudine alone in achieving a sustained response in patients with HBeAg-positive or -negative chronic hepatitis B, according to the results of two pivotal randomized, partially-blind, multicentre trials.
In HBeAg-positive disease, the proportion of patients with HBeAg sero-conversion or HBV DNA suppression to <100 000 copies/mL 24 weeks post-treatment was significantly higher among recipients of peginterferon-α-2a (40 kD) with or without lamivudine than in recipients of lamivudine alone. In addition, significantly more patients receiving peginterferon-α-2a (40 kD) with or without lamivudine versus lamivudine alone achieved a combined response or HBsAg seroconversion; however, there were no significant between-group differences in the histological response rate. Among peginterferon-α-2a (40 kD) monotherapy recipients who participated in a long-term follow-up study, the HBeAg seroconversion rate was 42% 1 year after the end of treatment, with 37% of patients achieving suppression of HBV DNA to <100 000 copies/mL.
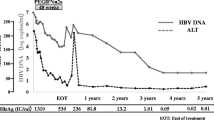
The trial in patients with HBeAg-negative chronic hepatitis B revealed that ALT normalization and HBV DNA suppression was seen in significantly more recipients of peginterferon-α-2a (40 kD) with or without lamivudine than in recipients of lamivudine alone, 24 weeks post-treatment. Moreover, significantly more peginterferon-α-2a (40 kD) monotherapy than lamivudine monotherapy recipients experienced a combined response, HBV DNA levels of <400 copies/mL, HBsAg loss or HBsAg seroconversion. In addition, significantly more patients receiving peginterferon-α-2a (40 kD) plus lamivudine than lamivudine monotherapy achieved a combined response or HBV DNA levels of <400 copies/mL. There were no significant between-group differences in histological response. Among patients who participated in a long-term follow-up study, an HBV DNA level of <10 000 copies/mL was achieved in 24% of patients who received peginterferon-α-2a (40 kD) with or without lamivudine after 4 years of follow-up; HBsAg loss occurred in 11% of patients receiving peginterferon-α-2a (40 kD) with or without lamivudine and in 2% of patients receiving lamivudine alone. In patients with HBeAg-negative disease who received peginterferon-α-2a (40 kD) with or without lamivudine, HBsAg clearance occurred in 12% of patients and inactive chronic hepatitis B occurred in 17% of patients, 5 years after the end of treatment.
Two studies compared peginterferon-α-2a (40 kD) with entecavir or adefovir dipivoxil in patients with HBeAg-positive chronic hepatitis B. After 48 weeks of therapy, rates of HBeAg and HBsAg seroconversion were significantly higher with peginterferon-a-2a (40 kD) than with entecavir; the on-treatment decline in HBeAg and HBsAg levels was also significantly greater with peginterferon-α-2a (40 kD) at weeks 24 and 48. There was no significant between-group difference in the proportion of patients with an HBV DNA level of <1000 copies/mL. In patients with HBeAg-positive chronic hepatitis B who were infected with lamivudine-resistant YMDD mutant strains of HBV, the HBeAg seroconversion rate at week 72 was significantly higher in patients receiving peginterferon-α-2a (40 kD) for 48 weeks than in those receiving adefovir dipivoxil for 72 weeks.
The addition of ribavirin or adefovir dipivoxil to peginterferon-α-2a (40 kD) therapy did not improve outcome in patients with HBeAg-negative chronic hepatitis B (assessed 24 weeks after the end of therapy).
Among patients with HBeAg-positive chronic hepatitis B, predictors of response to peginterferon-α therapy included female sex, high baseline ALT levels, low baseline HBV DNA levels and HBV genotype A infection. In this patient group, quantifying HBeAg levels after 24 weeks of peginterferon-α-2a (40 kD) therapy may be able to predict the absence of a response and provide sufficient information to decide if prematurely discontinuing therapy is justified (e.g. lower serum levels of HBeAg were associated with a greater likelihood of HBeAg sero-conversion). In addition, on-treatment HBsAg levels may also be useful as an early indicator of a sustained off-treatment response.
In patients with HBeAg-negative chronic hepatitis B, factors predictive of a sustained combined response 24 weeks after the end of peginterferon-α-2a (40 kD) therapy included high baseline ALT levels, low baseline HBV DNA levels, younger age, female sex, HBV genotype and type of treatment. HBV genotype and type of treatment remained predictive of a combined response to treatment 1 year after the end of therapy. In addition, suppression of HBV DNA levels to ≤400 copies/mL 6 months post-treatment was a good predictor of response 3 years post-treatment. Quantifying HBsAg levels may help identify which patients with HBeAg-negative chronic hepatitis B are likely to achieve a sustained response to peginterferon-α-2a (40 kD) therapy and which patients may benefit from a longer treatment duration.
Tolerability
In patients with HBeAg-negative or -positive chronic hepatitis B, the incidence of adverse events was higher in patients receiving peginterferon-α-2a (40 kD) alone or in combination with lamivudine than in those receiving lamivudine alone. The most commonly reported adverse events in recipients of subcutaneous peginterferon-α-2a (40 kD) 180 mg once weekly were typical of those seen with α-interferons and included pyrexia, fatigue, headache, myalgia, decreased appetite and alopecia. The proportion of patients with HBeAg-positive disease who discontinued therapy because of a safety reason was 3%, 4% and 1% among recipients of peginterferon-α-2a (40 kD) alone, peginterferon-α-2a (40 kD) plus lamivudine and lamivudine alone, respectively; rates in the corresponding treatment groups among patients with HBeAg-negative disease were 7%, 4% and 0%.
Peginterferon-α-2a (40 kD) dose modification was required in almost half of the patients who received peginterferon-α-2a (40 kD) with or without lamivudine (46–48% of patients). A laboratory abnormality (e.g. neutropenia, thrombocytopenia, elevated ALT levels) was the most common reason for dose modification (36–38% of patients) with adverse events accounting for dose modification in 7-13% of patients.
Pharmacoeconomic Considerations
Mixed results were seen in pharmacoeconomic analyses involving patients with HBeAg-negative or -positive chronic hepatitis B, with incremental cost-effectiveness ratios for peginterferon-α-2a (40 kD) versus lamivudine falling below commonly accepted cost-effectiveness thresholds in most, but not all, analyses.





Similar content being viewed by others
References
Lok ASF, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology 2009 Sep; 50(3): 1–36
Sorrell MF, Belongia EA, Costa J. National Institutes of Health consensus development conference statement: management of hepatitis B. Hepatology 2009 May; 49 (5 Suppl.): S4–12
Liaw Y-F, Leung N, Kao J-H, et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2008 update. Hepatol Int 2008; 2(3): 263–83
McMahon BJ.The natural history of chronic hepatitis B virus infection. Hepatology 2009 May; 49 (5 Suppl.): S45–55
European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of chronic hepatitis B. J Hepatol 2009 Feb; 50(2): 227–42
Roche. Pegasys (peginterferon alfa-2a): scientific discussion document [online]. Available from URL: http://www.emea.europa.eu/humandocs/PDFs/EPAR/pegasys/199602en6.pdf [Accessed 2009 Jun 10]
Liang TJ. Hepatitis B: the virus and disease. Hepatology 2009 May; 49 (5 Suppl.): S13–21
Harris JM, Martin NE, Modi M. Pegylation: a novel process for modifying pharmacokinetics. Clin Pharmacokinet 2001; 40(7): 539–51
Hoffmann-La Roche Inc. Pegasys® (peginterferon alfa-2a): US prescribing information [online]. Available from URL: http://www.rocheusa.com/products/pegasys/pi.pdf [Accessed 2009 Jun 10]
Perrillo R. Benefits and risks of interferon therapy for hepatitis B. Hepatology 2009 May; 49 (5 Suppl.): S103–11
Colombatto P, Civitano L, Bizzarri R, et al. A multiphase model of the dynamics of HBV infection in HBeAg-negative patients during pegylated interferon-α2a, lamivudine and combination therapy. Antivir Ther 2006; 11(2): 197–212
Marcellin P, Lau GKK, Bonino F, et al. Peginterferon alfa-2a alone, lamivudine alone, and the two in combination in patients with HBeAg-negative chronic hepatitis B. N Engl J Med 2004 Sep 16; 351(12): 1206–17
Zhang H-Y, Li J, Hui C-K, et al. Inhibition of viral replication with pegylated interferon alpha-2a increases IL-17 in chronic hepatitis B [abstract no. 934]. Hepatology 2007 Oct; 46 (4 Suppl. 1): 652
Roche. Pegasys (peginterferon alfa-2a): EU summary of product characteristics [online]. Available from URL: http://www.emea.europa.eu/humandocs/PDFs/EPAR/pegasys/H-395-PI-en.pdf [Accessed 2009 Jun 10]
Sulkowski M, Wright T, Rossi S, et al. Peginterferon alfa-2a does not alter the pharmacokinetics of methadone in patients with chronic hepatitis C undergoing methadone maintenance therapy. Clin Pharmacol Ther 2005 Mar; 77(3): 214–24
Cooksley WG, Piratvisuth T, Lee SD, et al. Peginterferon alpha-2a (40 kDa): an advance in the treatment of hepatitis B e antigen-positive chronic hepatitis B. J Viral Hepat 2003 Jul; 10(4): 298–305
Lau GKK, Piratvisuth T, Luo KX, et al. Peginterferon alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B. N Engl J Med 2005 Jun 30; 352(26): 2682–95
Chen X-F, Chen X-P, Huang J, et al. Comparison of peginterferon alfa-2a versus entecavir in patients with HBeAg-positive chronic hepatitis B with mildly elevated alanine aminotransferase levels [abstract]. 44th Annual Meeting of the European Association for the Study of the Liver; 2009 Apr 22-26; Copenhagen
Hou J, Sun J, Xie Q, et al. Efficacy and safety of peginterferon alfa-2a versus adefovir dipivoxil in treating lamivudine resistant HBeAg positive CHB: an interim analysis of a prospective randomized study [abstract no 978]. Hepatology 2008 Oct; 48(4): 745–6
Chen XP, Chen X-F, Huang J, et al. Extending peginterferon alfa-2a therapy in patients with HBeAg-positive chronic hepatitis B who did not achieve a response at week 48 can lead to HBeAg seroconversion and HBsAg clearance [abstract no. 410]. Hepatology 2009 Oct; 50 (4 Suppl.): 499A
Hou J, Sun J, Xie Q, et al. On-treatment quantification of HBsAg in difficult-to-treat patients with lamivudine resistance can help identify those most likely to acheive sustained post-treatment response to peginterferon alfa-2a rescue therapy [abstract no. 392]. Hepatology 2009 Oct; 50 (4 Suppl.): 490A
Lau GKK, Piratvisuth T, Luo KX, et al. Durability of response and occurrence of late response to peginterferon alpha-2a (40KD) [Pegasys] one year post-treatment in patients with HBeAg-positive chronic hepatitis B [abstract no. 50]. J Hepatol 2006 Jan 1; 44 (Suppl. 2): 23–4
Piratvisuth T, Lau G, Chao Y-C. Sustained response to peginterferon alfa-2a (40 kD) with or without lamivudine in Asian patients with HBeAg-positive and HBeAg-negative chronic hepatitis B. Hepatol Int 2008; 2: 102–10
Xu D-Z, Xie Y, Wei L, et al. Efficacy and safety of peginterferon alfa-2a in Chinese patients with HBeAg-positive chronic hepatitis B [abstract]. 43rd Annual Meeting of the European Association for the Study of the Liver; 2008 Apr 23–27; Milan
Zhu Y-Y, Dong J, Chen Y-T, et al. Extended treatment with peginterferon alfa-2a benefits patients with HBeAg-positive chronic hepatitis B with a partial response after 48 weeks of therapy: clinical experience from a Chinese center [abstract no. 453]. Hepatology 2009 Oct; 50 (4 Suppl.): 519A
Zhu Y-Y, Dong J, Chen Y-T, et al. Extending the treatment duration of peginterferon alfa-2a therapy to 72 weeks increases the rate of HBEAG seroconversion in patients with HBEAG-positive chronic hepatitis B [abstract]. 44th Annual Meeting of the European Association for the Study of the Liver; 2009 Apr 22–26; Copenhagen
Suh D, Lee HC, Byun K, et al. A multicenter, open-label study of efficacy and safety of peginterferon alfa-2a (40KD) in Korean patients with HBeAg-positive CHB harboring lamivudine-resistant YMDD mutants [abstract no. 981]. Hepatology 2008 Oct; 48 (4 Suppl.): 747
Rezzonico L, Galdame O, Frider B, et al. Twenty-four weeks therapy with peginterferon alfa-2a is similar to 48 weeks therapy in patients with HBeAg positive chronic hepatitis B and good predictors of response [abstract no. 978]. Hepatology 2007 Oct; 46 (4 Suppl. 1): 673
Hui C-K, Lai LSW, Lam P, et al. 48 weeks pegylated interferon alpha-2a is superior to 24 weeks of pegylated interferon alpha-2b in achieving hepatitis B e antigen seroconversion in chronic hepatitis B infection. Aliment Pharmacol Ther 2006 Apr 15; 23(8): 1171–8
Janssen HL, Rijckborst V, Cakaloglu Y, et al. 48 weeks of peginterferon alfa-2a alone or in combination with ribavirin for HBeAg-negative chronic hepatitis B: addition of ribavirin does not improve response rates [abstract no. 991]. Hepatology 2008 Oct; 48 (4 Suppl.): 752
Piccolo P, De Melia L, Bandiera F, et al. Peginterferon alpha-2a plus adefovir dipivoxil vs peginterferon alpha-2a monotherapy for 48 weeks in HBeAg-negative chronic hepatitis B: final results of the PEG for B randomized multicenter trial [abstract no. 256]. Gastroenterology 2008 Apr; 134 (4 Suppl. 1): 760–1
Gish RG, Lau DT-Y, Schmid P, et al. A pilot study of extended duration peginterferon alfa-2a for patients with hepatitis B e antigen-negative chronic hepatitis B. Am J Gastroenterol 2007 Dec; 102(12): 2718–23
Marcellin P, Bonino F, Lau GKK, et al. Sustained response of hepatitis B e antigen-negative patients 3 years after treatment with peginterferon alfa-2a. Gastroenterology 2009 Jun; 136(7): 2169–79
Marcellin P, Piratvisuth T, Brunetto M, et al. Virological and biochemical response in patients with HBeAg-negative chronic hepatitis B treated with peginterferon alfa-2a (40kD) with or without lamivudine: results of 4-year follow-up [abstract]. 43rd Annual Meeting of the European Association for the Study of the Liver; 2008 Apr 23–27; Milan
Marcellin P, Piratvisuth T, Brunetto MR, et al. A finite course of peginterfeon alfa-2a results in inactive chronic hepatitis B and HBsAg clearance 5 years post-treatment in patients with HBeAg-negative disease: baseline characteristics and predictive factors of long-term response [abstract no. 387]. Hepatology 2009 Oct; 50 (4 Suppl.): 487A
Fried MW, Piratvisuth T, Lau GKK, et al. HBeAg and hepatitis B virus DNA as outcome predictors during therapy with peginterferon alfa-2a for HBeAg-positive chronic hepatitis B. Hepatology 2008 Feb; 47(2): 428–34
Lau GK, Marcellin P, Brunetto M, et al. On-treatment HBsAg decline during peginterferon alfa-2a (40KD)-lamivudine in patients with HBeAg-positive CHB as a potential predictor of durable off-treatment response [abstract no. 910]. Hepatology 2008 Oct; 48 (4 Suppl.): 714
Lau GKK, Marcellin P, Brunetto M, et al. On-treatment monitoring of HBsAg levels to predict response to peginterferon alfa-2a in patients with HBeAg-positive chronic hepatitis B [abstract]. 44th Annual Meeting of the European Association for the Study of the Liver; 2009 Apr 22–26; Copenhagen
Piratvisuth T, Lau GKK, Marcellin P, et al. Association between HBeAg seroconversion and sustained HBV-DNA suppression in patients treated with peginterferon alpha-2a (40KD) (Pegasys) for HBeAg-positive chronic hepatitis B (CHB) [abstract no. 49]. J Hepatol 2006 Apr; 44 Suppl. 2: 23
Buster EH, Hansen BE, Lau GK, et al. Factors that predict response of patients with hepatitis B e antigen-positive chronic hepatitis B to peginterferon-alfa. Gastroenterology. Epub 2009 Sep 6
Janssen HLA, van Zonneveld M, Senturk H, et al. Pegylated interferon alfa-2b alone or in combination with lamivudine for HBeAg-positive chronic hepatitis B: a randomised trial. Lancet 2005 Jan 8; 365(9454): 123–9
Brunetto MR, Moriconi F, Bonino F, et al. Hepatitis B virus surface antigen levels: a guide to sustained response to peginterferon alfa-2a in HBeAg-negative chronic hepatitis B. Hepatology 2009 Apr; 49(4): 1141–50
Bonino F, Marcellin P, Lau GKK, et al. Predicting response to peginterferon α-2a, lamivudine and the two combined for HBeAg-negative chronic hepatitis B. Gut 2007 May; 56(5): 699–705
Brunetto M, Bonino F, Marcellin P, et al. Kinetics of HBsAg decline in patients with HBeAg-negative chronic hepatitis B treated with peginterferon alfa-2A according to genotype and its association with sustained HBsAg clearance 4 years post-treatment [abstract no. 965]. Hepatology 2008 Oct; 48 (4 Suppl.): 740
Marcellin P, Brunetto M, Bonino F, et al. In patients with HBeAg-negative chronic hepatitis B HBsAg serum levels early during treatment with peginterferon alfa-2a predict HBsAg clearance 4 years post-treatment [abstract no. 919]. Hepatology 2008 Oct; 48 (4 Suppl.): 718–9
Brunetto MR, Marcellin P, Bonino F, et al. HBsAg decline in HBeAg-negative patients treated with peginterferon alfa-2a is associated with sustained response up to 5 years post-treatment: patients with continuous HBsAg decline starting before week 24 achieve highest rates of response [abstract no. 452]. Hepatology 2009 Oct; 50 (4 Suppl.): 519A
Rijckborst V, ter Borg MJ, Akarca US, et al. Early reduction of serum HBsAg levels in HBeAg-negative chronic hepatitis B patients achieving sustained virological response after peginterferon alfa-2A +/- ribavirin treatment [abstract no. 986]. Hepatology 2008 Oct; 48 (4 Suppl.): 749–50
Rijckborst V, Hanesen BE, ter Borg M, et al. Mutations in the precore and basal core promoter regions do not influence responsiveness to pegylated interferon alfa-2a treatment for HBeAg-negative chronic hepatitis B [abstract no 497]. Hepatology 2009 Oct; 50(4): 542A
Rijckborst V, Hansen BE, Tabak F, et al. On-treatment prediction of sustained response in HBeAg-negative chronic hepatitis B patients treated with pegylated interferon alfa-2a [abstract no. 492]. Hepatology 2009 Oct; 50 (4 Suppl.): 539A
Moucari R, Mackiewicz V, Lada O, et al. Early serum HBsAg drop: a strong predictor of sustained virological response to pegylated interferon alfa-2a in HBeAg-negative patients. Hepatology 2009 Apr; 49(4): 1151–7
Xu D, Chen X, Zhang W. Response patterns of Chinese patients with chronic hepatitis B who achieved HBsAg clearance through treatment with peginterferon alfa-2a [abstract no. 953]. Hepatology 2008 Oct; 48 (4 Suppl.): 734
Brunetto M, Cavallone D, Moriconi F, et al. Kinetics of HBsAg decline during and following treatment of CHB: early and rapid HBsAg decline during peginterferon alfa-2A is predictive of HBsAg clearance [abstract no. 917]. Hepatology 2008 Oct; 48 (4 Suppl.): 717–8
Lu L, Ye D, Wang Y, et al. Correlation between HBV cccDNA and HBsAg levels and their reduction by peginterferon alfa-2a-based therapy in patients with chronic hepatitis B [abstract no. 979]. Hepatology 2008 Oct; 48 (4 Suppl.): 746
Takkenberg B, Zaaijer HL, de Niet A, et al. Baseline HBsAg levels predict HBsAg loss in HBeAg negative but not in HBeAg positive chronic hepatitis B patients treated with peginterferon alfa-2a (Pegasys®) and adefovir (HEPSERA): an interim analysis [abstract no. 488]. Hepatology 2009 Oct; 50 (4 Suppl.): 536A
Takkenberg B, Zaaijer HL, de Niet A, et al. High levels of HBsAg and HBV DNA during treatment predict failure for HBeAg seroconversion in HBeAg positive chronic hepatitis B patients treated with peginterferon alfa-2a (Pegasys®) and adefovir (HEPSERA): an interim analysis [abstract no. 495]. Hepatology 2009 Oct; 50 (4 Suppl.): 541A
Takkenberg B, Zaaijer HL, de Niet A, et al. End of treatment intrahepatic hepatitis B (HBV) covalently close circular DNA (cccDNA) predicts sustained virological response in chronic hepatitis B (CHB) patients treated with peginterferon alfa-2a and adefovir [abstract no. 486]. Hepatology 2009 Oct; 50 (4 Suppl.): 535A
Marcellin P, Lau GK, Zeuzem S, et al. Comparing the safety, tolerability and quality of life in patients with chronic hepatitis B vs chronic hepatitis C treated with peginterferon alpha-2a. Liver Int 2008 Apr; 28(4): 477–85
Pockros PJ, Carithers R, Desmond P, et al. Efficacy and safety of two-dose regimens of peginterferon alpha-2a compared with interferon alpha-2a in chronic hepatitis C: a multicenter, randomized controlled trial. Am J Gastro-enterol 2004 Jul; 99(7): 1298–305
Reddy KR, Wright TL, Pockros PJ, et al. Efficacy and safety of pegylated (40-kd) interferon α-2a compared with interferon α-2a in noncirrhotic patients with chronic hepatitis C. Hepatology 2001 Feb; 33(2): 433–8
Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med 2002 Sep 26; 347(13): 975–82
Heathcote EJ, Shiffman ML, Cooksley WG. Peginterferon alfa-2a in patients with chronic hepatitis C and cirrhosis. N Engl J Med 2000 Dec 7; 343(23): 1673–80
Zeuzem S, Feinman SV, Rasenack J. Peginterferon alfa-2a in patients with chronic hepatitis C. N Engl J Med 2000 Dec 7; 343(23): 1666–72
Veenstra DL, Sullivan SD, Lai M-Y, et al. HBeAg-negative chronic hepatitis B: cost-effectiveness of peginterferon alfa-2a compared to lamivudine in Taiwan. Value Health 2008 Mar–Apr; 11(2): 131–8
Sullivan SD, Veenstra DL, Chen P-J, et al. Cost-effectiveness of peginterferon alfa-2a compared to lamivudine treatment in patients with hepatitis B e antigen positive chronic hepatitis B in Taiwan. J Gastroenterol Hepatol 2007 Sep; 22(9): 1494–9
Veenstra DL, Sullivan SD, Dusheiko GM, et al. Cost-effectiveness of peginterferon α-2a compared with lamivudine treatment in patients with HBe-antigen-positive chronic hepatitis B in the United Kingdom. Eur J Gastroenterol Hepatol 2007 Aug; 19(8): 631–8
Lacey L, Chien R-N, Chuang W-L, et al. Economic evaluation of chronic hepatitis B treatments in Taiwan. J Gastroenterol Hepatol 2008 Apr; 23(4): 571–9
Lacey LF, Gane E. The cost-effectiveness of long-term antiviral therapy in the management of HBeAg-positive and HBeAg-negative chronic hepatitis B in Singapore. J Viral Hepat 2007 Nov; 14(11): 751–66
Dienstag JL. Benefits and risks of nucleoside analog therapy for hepatitis B. Hepatology 2009 May; 49 (5 Suppl.): S112–21
Ghany MG, Doo EC. Antiviral resistance and hepatitis B therapy. Hepatology 2009 May; 49 (5 Suppl.): S174–84
Fontana RJ. Side effects of long-term oral antiviral therapy for hepatitis B. Hepatology 2009 May; 49 (5 Suppl.): S185–95
Feld JJ, Wong DKH, Heathcote EJ. Endpoints of therapy in chronic hepatitis B. Hepatology 2009 May; 49 (5 Suppl.): S96–102
Andersson KL, Chung RT. Monitoring during and after antiviral therapy for hepatitis B. Hepatology 2009 May; 49 (5 Suppl.): S166–73
Perrillo RP. Hepatitis B surface antigen quantification as a current-day paradox: obtaining the gold in the face of diminishing returns. Hepatology 2009 Apr; 49(4): 1063–5
Wong GL-H, Chan HL-Y. Predictors of treatment response in chronic hepatitis B. Drugs 2009; 69(16): 2167–77
Degertekin B, Lok ASF. Indications for therapy in hepatitis B. Hepatology 2009 May; 49 (5 Suppl.): S129–37
Cooksley WGE. Peginterferon-α2a for the treatment of hepatitis B infection. Expert Opin Pharmacother 2005 Jul; 6(8): 1373–80
Hoffmann-La Roche. A study of Pegasys (peginterferon alfa-2a [40kD]) in patients with HBeAg positive chronic hepatitis B (CHB) [ClinicalTrials.gov identifier NCT00435825]. US National Institutes of Health, ClinicalTrials.gov [online]. Available from URL: http://clinicaltrials.gov [Accessed 2009 Aug 13]
Terrault NA. Benfits and risks of combination therapy for hepatitis B. Hepatology 2009 May; 49 (5 Suppl.): S122–8
Schering Corporation. Peglntron® (peginterferon alfa-2b): US prescribing information [online]. Available from URL: http://www.spfiles.com/pipeg-intron.pdf [Accessed 2009 Jun 17]
European Medicines Agency. PegIntron: EU summary of product characteristics [online]. Available from URL: http://www.emea.europa.eu/humandocs/PDFs/EPAR/Pegintron/H-280-PI-en.pdf [Accessed 2009 Jun 17]
Liu C-J, Chuang W-L, Lee C-M, et al. HBsAg clearance continues to increase post-treatment in patients with HCV/HBV coinfection treated with peginterferon alfa-2a plus ribavirin: 1.5 year follow-up [abstract no. 419]. Hepatology 2009 Oct; 50 (4 Suppl.): 503A
Kaiser S, Lutze B, Werner CR, et al. Extended treatment of HBV/HDV coinfection with peginterferon alpha 2a and adefovir or entecavir for 96 weeks versus 48 weeks leads to higher SVR rates in HDV viral replication [abstract no. 984]. Hepatology 2008 Oct; 48 (4 Suppl.): 748–9
Wedemeyer H, Yurdaydin C, Zachou K, et al. Pegylated interferon-alfa-2a plus adefovir combination therapy is superior to pegylated interferon-alfa-2a alone or adefovir monotherapy in reducing HBsAg levels in HDV-coinfected patients with low HBV viremia [abstract no. 981]. Hepatology 2006 Oct; 44 (4 Suppl. 1): 553–4
Sherman M, Shafran S, Burak K, et al. Management of chronic hepatitis C: consensus guidelines. Can J Gastroenterol 2007 Jun; 21 Suppl. C: 25–34C
Janssen HLA, Buster EHCJ. Comments on the EASL practice guidelines for the management of chronic hepatitis B: controversies in interferon-based therapy [letter]. J Hepatol 2009 Jul; 51(1): 224–6
Author information
Authors and Affiliations
Corresponding author
Additional information
Various sections of the manuscript reviewed by: F. Bonino, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico, Policlinico di Milano, Milan, Italy; S.J. Hadziyannis, Department of Medicine and Hepatology, Henry Dunant Hospital, Athens, Greece; N. Leung, Department of Medicine, Alice Ho Miu Ling Nethersole Hospital, Taipo, Hong Kong; M.B. Wan, Department of Infectious Diseases, Changhai Hospital, Second Military Medical University, Shanghai, China; C. Yurdaydin, Gastroenterology Department, University of Ankara Medical School, Ankara, Turkey.
Data Selection
Sources: Medical literature published in any language since 1980 on ‘peginterferon alfa 2a’, identified using MEDLINE and EMBASE, supplemented by AdisBase (a proprietary database). Additional references were identified from the reference lists of published articles. Bibliographical information, including contributory unpublished data, was also requested from the company developing the drug.
Search strategy: MEDLINE, EMBASE and AdisBase search terms were (‘peginterferon alfa 2a’ or ‘PEG-IFN alfa 2a’ or ‘peg-interferon alfa 2a’ or ‘pegylated interferon alfa 2a’) and ‘hepatitis B’. Searches were last updated 9 November 2009.
Selection: Studies in patients with chronic hepatitis B who received peginterferon-α-2a (40 kD). Inclusion of studies was based mainly on the methods section of the trials. When available, large, well controlled trials with appropriate statistical methodology were preferred. Relevant pharmacodynamic and pharmacokinetic data are also included.
Index terms: Peginterferon-α-2a (40 kD), chronic hepatitis B, pharmacodynamics, pharmacokinetics, therapeutic use, tolerability, pharmacoeconomics.
Rights and permissions
About this article
Cite this article
Keating, G.M. Peginterferon-α-2a (40 kD). Drugs 69, 2633–2660 (2009). https://doi.org/10.2165/11203660-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11203660-000000000-00000