Abstract
Objective: To assess the comparative tolerability of nebivolol and other cardioselective β-blockers (CSBs) in the treatment of mild-to-moderate hypertension.
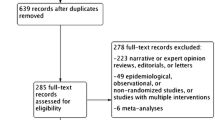
Data source: Database searches of MEDLINE, EMBASE, and the Cochrane Controlled Trials Register up to August 2004 identified randomised clinical trials comparing nebivolol and other CSBs in patients with mild-to-moderate hypertension, with a treatment duration of at least 4 weeks and reporting any tolerability data.
Design and methods: Baseline and post-intervention data on tolerability and efficacy were extracted from the studies. Tolerability was assessed by the rate of patients with adverse events (AEs), the total number of AEs and the number of drug-related AEs. Efficacy endpoints were the changes from baseline in diastolic and systolic blood pressure and the rate of treatment responders. For meta-analysis of data presenting relative frequency or means and standard deviations, the random effects model for proportions and means was applied. Absolute frequency data were normalised to the expected frequencies in 1000 patients and analysed by methods for comparison of Poisson distributed data. The quality of each trial was evaluated using a pre-defined checklist reflecting the basic potency of a selected study to detect the relative tolerability of the drugs investigated. The final analysis was performed in the high-quality studies; the low-quality studies were included, where appropriate, in robustness analysis.
Results: Ten trials comparing nebivolol with the CSBs atenolol, metoprolol or bisoprolol were selected. According to the pre-defined criteria, three studies were assessed as high-quality studies and seven as low-quality studies. The ratio of the rates of patients with AEs treated with nebivolol compared with those treated with the CSBs was 0.63 (95% CI 0.44, 0.91). The difference of the rates of patients with AEs reached −12.8% (95% CI −23.3%, −2.3%). The ratio of normalised total AEs was 0.71 (95% CI 0.62, 0.82), and the ratio of normalised drug-related AEs was 0.38 (95% CI 0.29, 0.50). In the expected frequencies of AEs in 1000 patients, the differences in number of normalised total AEs and drug-related AEs with nebivolol compared with the other CSBs were −147 (95% CI −205, −89) and −126 (95% CI −159, −93), respectively. In the efficacy meta-analysis, nebivolol was at least as effective as the other CSBs.
Conclusions: This meta-analysis revealed that the tolerability of nebivolol is superior to that of atenolol, metoprolol and bisoprolol, and confirmed that nebivolol is at least as effective as these CSBs in the treatment of mild-to-moderate hypertension. The treatment of mild-to-moderate essential hypertension with antihypertensive drugs that have a relatively favourable tolerability profile, such as nebivolol, may be a way to improve persistence with antihypertensive treatment and overcome the consequences of poor compliance.








Similar content being viewed by others
References
Blood Pressure Lowering Treatment Trialists’ Collaboration. ACE inhibitors, calcium antagonists and other blood-pressure-lowering drugs: results of prospectively designed overviews of randomized trials. Lancet 2000; 356: 1955–64
European Society of Hypertension-European Society of Cardiology Guidelines Committee. 2003 European Society of Hypertension-European Society of Cardiology guidelines for the management of arterial hypertension [published erratum appears in J Hypertens 2003 Nov; 21 (11): 2203–4]. J Hypertens 2003 Jun; 21 (6): 1011–53
Mancia G, Grassi G. The hypertension guidelines of the Seventh Joint National Committee: a critical review. High Blood Press Cardiovasc Prev 2004; 11(2): 55–9
Krousel-Wood M, Thomas S, Muntner P, et al. Medication adherence: a key factor in achieving blood pressure control and good clinical outcomes in hypertensive patients. Curr Opin Cardiol 2004 Jul; 19(4): 357–62
Gascon JJ, Sanchez-Ortuno M, Llor B, et al. Why hypertensive patients do not comply with the treatment: results from a qualitative study. Fam Pract 2004 Apr; 21(2): 125–30
Burnier M, Santschi V, Favrat B, et al. Monitoring compliance in resistant hypertension: an important step in patient management. J Hypertens 2003 May; 21Suppl. 2: S37–42
Hajjar I, Kotchen TA. Trends in prevalence, awareness, treatment, and control of hypertension in the United States, 1988-2000. JAMA 2003 Jul 9; 290(2): 199–206
Dusing R. Adverse events, compliance, and changes in therapy. Curr Hypertens Rep 2001; 3: 488–92
Mancia G, Seravalle G, Grassi G. Tolerability and treatment compliance with angiotensin II receptor antagonists. Am J Hypertens 2003; 16: 1066–73
Kuroedov A, Cosentino F, Luscher TF. Pharmacological mechanisms of clinically favorable properties of a selective β1-adrenoceptor antagonist, nebivolol. Cardiovasc Drug Rev 2004; 22(3): 155–68
Cockcroft JR, Chowienczyk PJ, Brett SE, et al. Nebivolol vasodilates human forearm vasculature: evidence for an L-arginine/NO-dependent mechanism. J Pharmacol Exp Ther 1995; 274: 1067–71
Nuttall SL, Routledge HC, Kendall MJ. A comparison of the β1-selectivity of three β1-selective beta-blockers. J Clin Pharm Ther 2003; 28: 179–86
Pessina AC. Metabolic effects and safety profile of nebivolol. J Cardiovasc Pharmacol 2001; 38Suppl. 3: S33–5
Leysen JE, Pauwels PJ, Gommeren W, et al. The receptor binding profile of the new antihypertensive agent nebivolol and its stereoisomers compared with various beta-adrenergic blockers. Drug Invest 1991; 3Suppl. 1: 120–1
Bowman AJ, Chen CP, Ford GA. Nitric oxide mediated venodilator effects of nebivolol. Br J Clin Pharmacol 1994; 38: 199–204
Van Nueten L, Rishoj NM, Vertommen C, et al. Nebivolol versus enalapril in essential hypertension: a long-term double-blind comparative trial. Acta Clin Belg 1999; 54: 19–25
van Merode T, Van Bortel LM, Smeets FA, et al. Verapamil and nebivolol improve carotid artery distensibility in hypertensive patients. J Hypertens 1989; 7Suppl. 6: S262–3
Lacourcière Y, Lefèvre J, Poirier L, et al. A double-blind crossover comparison of nebivolol and lisinopril in the treatment of ambulatory hypertension. Am J Ther 1994; 1: 74–80
Lacourcière Y, Poirier L, Lefebvre J, et al. Comparative effects of a new cardioselective beta-blocker nebivolol and nifedipine sustained-release on 24-hour ambulatory blood pressure and plasma lipoproteins. J Clin Pharmacol 1992; 32: 660–6
Rosei EA, Rizzoni D, Comini S, et al. Evaluation of the efficacy and tolerability of nebivolol versus lisinopril in the treatment of essential arterial hypertension: a randomized, multicentre, double-blind study. Blood Press 2003; 12Suppl. 1: 30–5
Van Nueten L, Lacourciére Y, Vyssoulis G, et al. Nebivolol versus nifedipine in the treatment of essential hypertension: a double-blind, randomized, comparative trial. Am J Ther 1998; 5: 237–43
Van Nueten L, Schelling A, Vertommen C, et al. Nebivolol vs enalapril in the treatment of essential hypertension: a double-blind randomised trial. J Hum Hypertens 1997; 11: 813–9
Mazza A, Gil-Extremera B, Maldonato A, et al. Nebivolol vs amlodipine as first-line treatment of essential arterial hypertension in the elderly. Blood Press 2002; 11: 182–8
Cockcroft J. Nebivolol: a review. Expert Opin Pharmacother 2004; 5: 893–9
McNeely W, Goa KL. Nebivolol in the management of essential hypertension: a review. Drugs 1999; 57: 633–51
Grassi G, Trevano FQ, Facchini A, et al. Efficacy and tolerability profile of nebivolol vs atenolol in mild-to-moderate essential hypertension: results of a double-blind randomized multicentre trial. Blood Press 2003; 12Suppl. 2: 35–40
Uhlír O, Fejfusa M, Havránek K, et al. Nebivolol versus metoprolol in the treatment of hypertension. Drug Invest 1991; 3Suppl. 1: 107–10
Van Nueten L, Taylor FR, Robertson JI. Nebivolol vs atenolol and placebo in essential hypertension: a double-blind randomised trial. J Hum Hypertens 1998; 12: 135–40
Czuriga I, Riecansky I, Bodnar J, et al. Comparison of the new cardioselective beta-blocker nebivolol with bisoprolol in hypertension: the Nebivolol, Bisoprolol Multicenter Study (NEBIS). Cardiovasc Drugs Ther 2003; 17: 257–63
Brophy JM, Joseph L, Rouleau JL. Beta-blockers in congestive heart failure: a Bayesian meta-analysis. Ann Intern Med 2001; 134: 550–60
Agusti A, Bonet S, Arnau JM, et al. Adverse effects of ACE inhibitors in patients with chronic heart failure and/or ventricular dysfunction: meta-analysis of randomised clinical trials. Drug Saf 2003; 26: 895–908
Carneiro AV. Measures of association in clinical trials: definition and interpretation. Rev Port Cardiol 2003; 22: 1393–401
Hintze JL. NCSS user’s guide [computer program]. Kaysville (UT): Number Cruncher Statistical Systems, 2003
Uitenbroek D. SISA (Simple Interactive Statistical Analysis) 1997 [online]. Available from URL: http://home.clara.net/sisa/index.htm [Accessed 2004 Oct]
Yaduvanshi A, Tyagi S, Rastogi V, et al. Evaluation of the efficacy and tolerability of nebivolol versus atenolol in the treatment of essential arterial hypertension: a randomized study [abstract]. Indian Heart J 2003; 55: 311
Buval’tsev VI, Spasskaia MB, Nebieridze DV, et al. Pharmacological modulation of NO synthesis in patients with arterial hypertension and endothelial dysfunction [in Russian]. Klin Med (Mosk) 2003; 81: 51–5
Fogari R, Zoppi A, Lazzari P, et al. Comparative effects of nebivolol and atenolol on blood pressure and insulin sensitivity in hypertensive subjects with type II diabetes. J Hum Hypertens 1997; 11: 753–7
Makolkin VI, Akhmedova OO, Buval’tsev VI, et al. Clinical and metabolic effects of cardioselective beta-adrenoblockers nebivolol and metoprolol in patients with hypertension and ischemic heart disease associated with type 2 diabetes [in Russian]. Kardiologiia 2003; 43: 40–3
Simon G, Johnson M. Comparison of antihypertensive and β1-adrenoceptor antagonist effect of nebivolol and atenolol in essential hypertension. Clin Exp Hypertens 1993; 15: 501–9
Vojacek J, Kolar K. Comparison of the effects of nebivolol and metoprolol on left ventricular diastolic function in patients with hypertension. Drug Invest 1991; 3Suppl. 1: 164–6
Ernest E, Pittler MH. Systematic reviews neglect safety issues. Arch Intern Med 2001; 161: 125–6
Ioannidis JPA, Lau J. Completeness of safety reporting in randomized trials: an evaluation of 7 medical areas. JAMA 2001; 285: 437–43
Ioannidis JPA, Lau J. Improving safety reporting from randomised trials. Drug Saf 2002; 25(2): 77–84
Acknowledgements
No sources of funding were used to assist in the preparation of this review. The authors have no conflicts of interest that are directly relevant to the content of this review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ambrosioni, E., Borghi, C. Tolerability of Nebivolol in Head-To-Head Clinical Trials Versus Other Cardioselective β-Blockers in the Treatment of Hypertension. High Blood Press Cardiovasc Prev 12, 27–35 (2005). https://doi.org/10.2165/00151642-200512010-00005
Published:
Issue Date:
DOI: https://doi.org/10.2165/00151642-200512010-00005