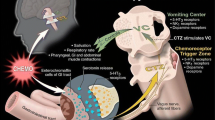
Abstract
The current standard of care with respect to preventing acute chemotherapy-induced nausea and vomiting (CINV) in children includes the administration of a 5-HT3 antagonist with or without a corticosteroid, depending on the emetogenicity of the chemotherapy to be given. Problems in assessing the emetogenicity of chemotherapy regimens and nausea severity in children may influence the degree of success of CINV prophylaxis. Nevertheless, the majority of children who receive chemotherapy today experience moderate to complete control of acute CINV when given appropriate antiemetic prophylaxis. If children vomit or experience nausea despite appropriate prophylaxis, then measures must be taken to treat these symptoms since these children are likely to go on to experience delayed or anticipatory CINV. However, appropriate selection of interventions to treat acute CINV in children is limited by the lack of rigorous evidence to support one approach over another. Lorazepam is suggested as an immediate agent for the treatment of acute CINV. Doses and frequencies of the 5-HT3 antagonist and corticosteroid administered for initial prophylaxis should also be maximized. Further treatment must be tailored to the circumstances and preferences of each child and family. Options include crossover to another 5-HT3 antagonist, or administration of an adjunctive antiemetic such as metopimazine, low dose metoclopramide, domperidone, alizapride, nabilone, scopolamine, prochlorperazine, or chlorpromazine. Complementary interventions such as acupuncture, hypnosis, counseling, or ginger may also be of benefit. Further study is required to establish optimal antiemetic strategies in children.





Similar content being viewed by others
References
Coates A, Abraham S, Kaye SB, et al. On the receiving end: patients’ perception of the side-effects of cancer chemotherapy. Eur J Cancer Clin Oncol 1983; 19: 203–8
Griffin AM, Butow PN, Coates AS, et al. On the receiving end V: patient perceptions of the side effects of cancer chemotherapy in 1993. Ann Oncol 1996; 7: 189–95
De Boer-Dennert M, de Wit R, Schmitz PIM, et al. Patient perceptions of the side-effects of chemotherapy: the influence of 5HT3 antagonists. Br J Cancer 1997; 76: 1055–61
American Society of Health System Pharmacists. ASHP therapeutic guidelines on the pharmacologic management of nausea and vomiting in adult and pediatric patients receiving chemotherapy or radiation therapy or undergoing surgery. Am J Health Syst Pharm 1999; 56: 729–64
Billett AL, Sallan SE. Antiemetics in children receiving cancer chemotherapy. Support Care Cancer 1994; 2: 279–85
Morrow GR, Roscoe JA, Hickok JT, et al. Nausea and emesis: evidence for a biobehavioral perspective. Support Care Cancer 2002; 10: 96–105
Tyc VL, Mulhern RK, Bierberich AA. Anticipatory nausea and vomiting in pediatric cancer patients: an analysis of conditioning and coping variables. J Dev Behav Pediatr 1997; 18: 27–33
Dupuis LL, Lau R, Greenberg ML. Effectiveness of strategies for the prevention of acute antineoplastic-induced nausea and vomiting in children with acute lymphoblastic leukemia. Can J Hosp Pharm 1999; 52: 350–61
Foot ABM, Hayes C. Audit of guidelines for effective control of chemotherapy and radiotherapy induced emesis. Arch Dis Child 1994; 71: 475–80
Passalacqua R, Cocconi G, Bella M, et al. Double-blind, randomized trial for the control of delayed emesis in patients receiving cisplatin: comparison of placebo vs adrenocorticotropic hormone (ACTH). Ann Oncol 1992; 3: 481–5
Shinkai T, Saijo N, Eguchi K, et al. Control of cisplatin-induced delayed emesis with metoclopramide and dexamethasone: a randomized controlled trial. Jpn J Clin Oncol 1989; 19: 40–4
Roila F, Boschetti E, Tonato M, et al. Predictive factors of delayed emesis in cisplatin-treated patients and tolerability of metoclopramide or dexamethasone. Am J Clin Oncol Cancer Clin Trials 1991; 14: 238–42
Gendera DR, Harvey WH, Monaghan GG, et al. The delayed emesis syndrome from cisplatin: phase III evaluation of ondansetron versus placebo. Semin Oncol 1992; 19Suppl. 10: 67–71
Kris MG, Gralla RH, Tyson LB, et al. Controlling delayed vomiting: double-blind randomized trial comparing placebo, dexamethasone alone and metoclopramide plus dexamethasone in patients receiving cisplatin. J Clin Oncol 1989; 7: 108–14
The Italian Group for Antiemetic Research. Delayed emesis induced by moderately emetogenic chemotherapy: do we need to treat all patients? Ann Oncol 1997; 8: 561–7
Pinkerton CR, Williams D, Wootton C, et al. 5-HT3 antagonist ondansetron: an effective outpatient antiemetic in cancer treatment. Arch Dis Child 1990; 65: 822–5
Dick GS, Meller ST, Pinkerton CR. Randomised comparison of ondansetron and metoclopramide plus dexamethasone for chemotherapy induced emesis. Arch Dis Child 1995; 73: 243–5
Dupuis LL, Lau R, Greenberg ML. Delayed nausea and vomiting in children receiving antineoplastics. Med Pediatr Oncol 2001; 37: 115–21
Hesketh PJ, Kris MG, Grunberg SM, et al. Proposal for classifying the acute emetogenicity of cancer chemotherapy. J Clin Oncol 1997; 15: 103–9
Small BE, Holdsworth MT, Raisch DW, et al. Survey ranking of emetogenic control in children receiving chemotherapy. J Pediatr Hematol Oncol 2000; 22: 125–32
Zeltzer LK, LeBaron S, Zeltzer PM. A prospective assessment of chemotherapy-related nausea and vomiting in children with cancer. Am J Pediatr Hematol Oncol 1984; 6: 5–16
Zeltzer L, LeBaron S, Zeltzer PM. The effectiveness of behavioral intervention for reduction of nausea and vomiting in children and adolescents receiving chemotherapy. J Clin Oncol 1984; 2: 683–90
Tyc VL, Mulhern RK, Fairclough D, et al. Chemotherapy induced nausea and emesis in pediatric cancer patients: external validity of child and parent emesis ratings. J Dev Behav Pediatr 1993; 14: 236–41
Koseoglu V, Kurekci AE, Atay AA, et al. Comparison of the efficacy and side-effects of ondansetron and metoclopramide-diphenhydramine administered to control nausea and vomiting in children treated with antineoplastic chemotherapy: a prospective randomized study. Eur J Pediatr 1998; 157: 806–10
Alvarez O, Freeman A, Bedros A, et al. Randomized double-blind crossover ondansetron-dexamethasone versus ondansetron-placebo study for the treatment of chemotherapy-induced nausea and vomiting in pediatric patients with malignancies. J Pediatr Hematol Oncol 1995; 17: 145–50
Zeltzer LK, LeBaron S, Richie DM, et al. Can children understand and use a rating scale to quantify somatic symptoms: assessment of nausea and vomiting as a model. J Consult Clin Psychol 1988; 56: 567–72
Hinds PS, Quargnenti AG, Wentz TJ. Measuring symptom distress in adolescents with cancer. J Pediatr Oncol Nurs 1992; 9: 84–6
DelFavero A, Roila F, Basurto C, et al. Assessment of nausea. Eur J Clin Pharmacol 1990; 38: 115–20
Rhodes VA, Watson PM, Johnson MH. Development of reliable and valid measures of nausea and vomiting. Cancer Nurs 1984; 7: 33–41
Hesketh PJ, Gralla RJ, de Bois A, et al. Methodology of antiemetic trials: response assessment, evaluation of new agents and definition of chemotherapy emetogenicity. Support Care Cancer 1998; 6: 221–7
Wong DL, Baker CM. Pain in children: comparison of assessment scales. Pediatr Nurs 1988; 14: 9–17
Wilcock L, Dupuis L. Preliminary evaluation of antiemetics in children undergoing bone marrow transplant conditioning [abstract]. Can J Hosp Pharm 1998; 51: 88
Antiemetic Subcommittee of the Multinational Association of Supportive Care in Cancer (MASCC). Prevention of chemotherapy- and radiotherapy-induced emesis: results of the Perugia consensus conference. Ann Oncol 1998; 9: 811–9
Gralla RJ, Osoba D, Kris MG, et al. Recommendations for the use of antiemetics: evidence-based, clinical practice guidelines. J Clin Oncol 1999; 17: 2971–94
Roila F, Aapro M, Stewart A. Optimal selection of antiemetics in children receiving cancer chemotherapy. Support Care Cancer 1998; 6: 215–20
Sanchez LA, Holdsworth M, Bartel SB. Stratified administration of serotonin 5-HT3 receptor antagonists (setrons) for chemotherapy-induced emesis. Pharm-acoeconomics 2000; 18: 533–56
Culy CR, Bhana N, Plosker GL. Ondansetron: a review of its use as an antiemeticin children. Pediatr Drugs 2001; 3: 441–79
Mabro M, Cohn R, Zanesco L, et al. Granisetron en solution buvable dans la prevention des vomissements chimio-induits de l’enfant: comparaison en double aveugle de deux posolgies. Bull Cancer 2000; 87: 259–64
Zoubek A, Kronberger M, Puschmann A, et al. Ondansetron in the control of chemotherapy-induced and radiotherapy-induced emesis in children with malignancies. Anticancer Drugs 1993; 2Suppl. 2: 17–21
Holdsworth MT, Raisch DW, Duncan MH, et al. Assessment of chemotherapy-induced emesis and evaluation of a reduced-dose intravenous ondansetron regimen in pediatric outpatients with leukemia. Ann Pharmacother 1995; 29: 16–21
Jacobson SJ, Shore RW, Greenberg M, et al. The efficacy and safety of granisetron in pediatric cancer patients who had failed standard antiemetic therapy during anticancer chemotherapy. Am J Pediatr Hematol Oncol 1994; 16: 231–5
Tsuchida Y, Hayashi Y, Asami K, et al. Effects of granisetron in children undergoing high-dose chemotherapy: a multi-institutional, cross-over study. Int J Oncol 1999; 14: 673–9
Robson H, Meyer S, Shalet S, et al. The anti-tumor effects of cytotoxics are modulated by glucocorticoids in human osteosarcoma in vitro [abstract]. Med Pediatr Oncol 1999; 33: 155
Robson H, Anderson E, Eden O, et al. Glucocorticoid pretreatment reduces the cytotoxic effects of a variety of DNA-damaging agents on rat tibial growth-plate chondrocytes in vitro. Cancer Chemother Pharmacol 1998; 42: 171–6
Mariotta M, Perewusnyk G, Koechli OR, et al. Dexamethasone-induced enhancement of resistance to ionizing radiation and chemotherapeutic agents in human tumor cells. Strahlenther Onkol 1999; 175: 392–6
Weiler M, Schmidt C, Roth W, et al. Chemotherapy of human malignant glioma: prevention of efficacy by dexamethasone? Neurology 1997; 48: 1704–9
Donelli MG, Zucchetti M, D’Incalci M. Do anticancer agents reach the tumor target in the human brain? Cancer Chemother Pharmacol 1992; 30: 251–60
Straathof CS, van den Bent MJ, Ma J, et al. The effect of dexamethasone on the uptake of cisplatin in 9L glioma and the area of brain around tumor. J Neurooncol 1998; 37: 1–8
The Italian Group for Antiemetic Research. Dexamethasone alone or in combination with ondansetron for the prevention of delayed nausea and vomiting induced by chemotherapy. N Engl J Med 2000; 342: 1554–9
Lazarus HM, Bryson JC, Lemon E, et al. Antiemetic efficacy and pharmacokinetic analyses of the serotonin antagonist ondansetron (GR 38032F) during multiple-day chemotherapy with cisplatin prior to autologous bone marrow transplantation. J Natl Cancer Inst 1990; 80: 1776–9
Grunberg SM, Groshen S, Robinson DC, et al. Correlation of anti-emetic efficacy and plasma levels of ondansetron. Eur J Cancer 1990; 26: 879–82
Seynaeve C, de Mulder P, van Liessum P, et al. A positive correlation of the plasma ondansetron level with the control of cisplatin induced emesis [abstract]. Ann Oncol 1990; 1 Suppl.: 112
Pritchard JF, Wells CD. Relationships between ondansetron systemic exposure and antiemetic efficacy and safety in cancer patients receiving cisplatin. Pharmacology 1992; 45: 188–94
Bryson JC, Pritchard JF, Shurin S, et al. Efficacy, pharmacokinetics and safety of ondansetron in pediatric chemotherapy patients [abstract]. Clin Pharmacol Ther 1991; 49: 161
Haberer LJ, Palmer JL. Evaluation of the exposure-response relationship for ondansetron in pediatric cancer patients [abstract]. Clin Pharmacol Ther 1995; 53: 156
Dixon CM, Colthup PV, Scrabjit-Singh CJ, et al. Multiple forms of cytochrome P450 are involved in the metabolism of ondansetron in humans. Drug Metab Dispos 1995; 23: 1225–30
Venkatakrishnan K, von Moltke LL, Greenblatt DJ. Human drug metabolism and the cytochromes P450: application and relevance of in vitro models. J Clin Pharmacol 2001; 41: 1149–79
Kaiser R, Sezer O, Papies A, et al. Patient-tailored antiemetic treatment with 5-hydroxytryptamine type 3 receptor antagonists according to cytochrome P-450 2D6 genotypes. J Clin Oncol 2002; 20: 2805–11
Carmichael J, Keizer HJ, Cupissol D, et al. Use of granisetron in patients refractory to previous treatment with antiemetics. Anticancer Drugs 1998; 9: 381–5
de Wit R, de Boer AC, vd Linden GHM, et al. Effective cross-over to granisetron after failure to ondansetron, a randomized double-blind study in patients failing ondansetron plus dexamethasone during the first 24 hours following highly emetogenic chemotherapy. Br J Cancer 2001; 85: 1099–101
Molino A, Guglielmo L, Azzolini ME, et al. The antiemetic activity of high-dose metoclopramide and high-dose alizapride in combination with lorazepam in patients receiving cancer chemotherapy: a prospective, randomized, double-blind study. Oncology 1991; 48: 111–5
Kris MG, Tyson LB, Gralla R. Extrapyramidal reactions with high-dose metoclopramide. N Engl J Med 1983; 305: 905–9
Howrie DL, Felix C, Wollman M, et al. Metoclopramide as an antiemetic agent in pediatric oncology patients. Drug Intell Clin Pharm 1986; 20(2): 122–4
Nahata MC, Ford C, Ruymann FB. Pharmacokinetics and safety of prochlorperazine in paediatric patients receiving cancer chemotherapy. J Clin Pharm Ther 1992; 17: 121–3
Allen JC, Gralla R, Reilly L, et al. Metoclopramide: dose-related toxicity and preliminary antiemetic studies in children receiving cancer chemotherapy. J Clin Oncol 1985; 3: 1136–41
Graham-Pole J, Weare J, Engel S, et al. Antiemetics in children receiving cancer chemotherapy: a double-blind prospective randomized study comparing metoclopramide with chlorpromazine. J Clin Oncol 1986; 4: 1110–3
Graham-Pole J, Engel S. Dose related extrapyramidal effect of metoclopramide in children receiving chemotherapy [abstract]. Proc Am Soc Clin Oncol 2002; 3: 104
Smith WL, Jackson CB, Sancilio LF, et al. Effect of diphenhydramine on the antiemetic action of metoclopramide against cisplatin-induced emesis in dogs. Cancer Treat Rep 1986; 70: 934–55
Tsavaris N, Zamanis N, Zinelis A, et al. Diphenhydramine for nausea and vomiting related to cancer chemotherapy with cisplatin. J Pain Symptom Manage 1991; 6: 461–5
Herrstedt J, Aapro MS, Smyth JF, et al. Corticosteroids, dopamine antagonists and other drugs. Support Care Cancer 1998; 6: 204–14
Osoba D, Warr DG, Fitch MI, et al. Guidelines for the optimal management of chemotherapy-induced nausea and vomiting: a consensus. Can J Oncol 1995; 5: 381–400
Antiemetic Subcommittee of the Multinational Association of Supportive Care in Cancer Prevention of Chemotherapy- and Radiotherapy-Induced Emesis. Results of the Perugia consensus conference. Ann Oncol 1998; 9: 811–9
Aapro MS. How do we manage patients with refractory or breakthrough emesis? Support Care Cancer 2002; 10: 106–9
Osborne RJ, Slevin ML, Hunter RW, et al. Cardiac arrhythmias during cytotoxic chemotherapy: role of domperidone. Hum Toxicol 1985; 4: 617–23
Cunningham D, Evans C, Gazet JC, et al. Comparison of antiemetic efficacy of domperidone, metoclopramide, and dexamethasone in patients receiving outpatient chemotherapy regimens. BMJ 1987; 295; 2: 250
Polubiec A, Krasnopolska-Polanczky Z, Duda-Krol W, et al. Evaluation of the antiemetic effect of domperidone in patients treated with cytostatic drugs. Ther Hung 1992; 40: 169–72
Dalzell AM, Bartlett H, Lilleyman JS. Nabilione: an alternative antiemetic for cancer chemotherapy. Arch Dis Child 1986; 61: 502–5
O’Meara A, Mott M. Domperidone as an antiemetic in paediatric oncology. Cancer Chemother Pharmacol 1981; 6: 147–9
Nguyen J, Sibille G, Cazassus F, et al. Manifestations extrapyramidales aigues apres prise de domperidone. Arch Pediatr 2000; 7: 1131–2
Bleiberg H, Gerard B, Dalesio O, et al. Activity of a new antiemetic agent: alizapride: a randomized double-blind crossover controlled trial. Cancer Chemother Pharmacol 1988; 22: 316–20
Basurto C, Roila F, Del Favero A, et al. A prospective randomized double-blind crossover study comparing the antiemetic activity of alizapride and metoclopramide in patients receiving cisplatin chemotherapy. Cancer Invest 1988; 6: 475–9
Depierre A, Lebeau B, d’Allens H. A comparison of ondansetron with alizapride plus methylprednisolone in the control of cisplatin-induced emesis: the French Pneumology Group for the Ondansetron Study. Oncology 1992; 49: 305–11
Rey E, Stettler E, D’athis P, et al. Pharmacokinetics of alizapride in children receiving chemotherapy for solid tumour. Fundam Clin Pharmacol 2001; 15: 217–20
Guitteny MA, Pajot C, Chalumeau M, et al. Traitement des vomissements induits par la chimiotherapie des cancers chez l’enfant. Arch Pediatr 1998; 5: 661–8
Carr BI, Bertrand M, Browning S, et al. A comparison of the antiemetic efficacy of prochlorperazine and metoclopramide for the treatment of cisplatin-induced emesis: a prospective, randomized, double-blind study. J Clin Oncol 1985; 3: 1127–32
Relling MV, Mulhern RK, Fairclough D, et al. Chlorpromazine with and without lorazepam as antiemetic therapy in children receiving uniform chemotherapy. J Pediatr 1993; 123: 811–6
Hahlen K, Quintana E, Pinkerton CR, et al. A randomized comparison of intravenously administered granisetron versus chlorpromazine plus dexameth-asone in the prevention of ifosfamide-induced emesis in children. J Pediatr 1995; 126: 309–13
Crucitt MA, Hyman W, Grote T, et al. Efficacy and tolerability of oral ondansetron versus prochlorperazine in the prevention of emesis associated with cyclophos-phamide-based chemotherapy and maintenance of health-related quality of life. Clin Therapeutics 1996; 18: 778–88
Nausea and vomiting (PDQ®) [online]. Available from URL: http://www.cancer.gov/cancerinfo/pdq/supportivecare/nausea/healthprofessional/#section_163 [Accessed 2003 Jul 29]
De Mulder PHM, Roila F, Kris MG, et al. Consensus regarding multiple day and rescue antiemetic therapy. Support Care Cancer 1998; 6: 248–52
Herrstedt J. Chemotherapy-induced nausea and vomiting with special emphasis on metopimazine. Dan Med Bull 1998; 45: 412–22
Herrstedt J, Sigsgaard T, Boesgaard M, et al. Ondansetron plus metopimazine compared with ondansetron alone in patients receiving moderately emetogenic chemotherapy. N Engl J Med 1993; 328: 1076–80
Herrstedt J, Sigsgaard T, Handberg J, et al. Randomized, double-blind comparison of ondansetron versus ondansetron plus metopimazine as antiemetic prophylaxis during platinum-based chemotherapy in patients with cancer. J Clin Oncol 1997; 15: 1690–6
Lebeau B, Depierre A, Giovannini M, et al. The efficacy of a combination of ondansetron, methylprednisolone and metopimazine in patients previously uncontrolled with a dual antiemetic treatment in cisplatin-based chemotherapy. The French Ondansetron Study Group. Ann Oncol 1997; 8: 887–92
Debray H, Guihard J, Peyramond D, et al. Treatment of vomiting in infants and children induced by acute infectious pathology: a comparative study of alizapride versus metopimazine. Ann Pediatr (Paris) 1990; 37: 683–7
Herrstedt J, Sigsgaard T, Angelo HR, et al. Dose-finding study of oral metopimazine. Support Care Cancer 1997; 5: 38–43
Tramer MR, Carroll D, Campbell A, et al. Cannabinoids for control of chemotherapy induced nausea and vomiting: quantitative systematic review. BMJ 2001; 323: 1–8
Chan HSL, Correia J, MacLeod SM. Nabilone versus prochlorperazine for control of cancer chemotherapy-induced emesis in children: a double-blind, crossover trial. Pediatrics 1987; 79: 946–52
Longo DL, Wesley M, Howser D, et al. Results of a randomized double-blind crossover trial of scopolamine versus placebo administered by transdermal patch for the control of cisplatin-induced emesis. Cancer Treat Rep 1982; 66: 1975–6
Malone JM, Christensen CW, Yashinsky D, et al. Prochlorperazine and transdermal scopolamine added to a metoclopramide antiemetic regimen. J Reprod Med 1990; 35: 932–4
Helson L. Total control of chemotherapy induced emesis. Anticancer Res 1992; 12: 2243–4
Meyer BR, O’Mara V, Reidenberg MM. A controlled clinical trial of the addition of transdermal scopolamine to a standard metoclopramide and dexamethasone antiemetic regimen. J Clin Oncol 1987; 5: 1994–7
Sawicka J, Sallan S. Transdermal therapeutic system-scopolamine (TS): prevention of vomiting associated with cancer chemotherapy (CC) [abstract]. Proc Am Soc Clin Oncol 1977; 18: 302
Parfitt A. Acupuncture as an antiemetic treatment. J Altern Complement Med 1996; 2: 167–74
Vickers AJ. Can acupuncture have specific effects on health: a systematic review of acupuncture antiemesis trials. J Royal Soc Med 1996; 89: 303–11
Acupuncture. NIH consensus statement 1997 Nov 3–5; 15(5): 1–34 [online]. Available from URL: http://consensus.nih.gov/cons/107/107_statement.htm [Accessed 2003 Aug 7]
Somri M, Vaida SJ, Sabo E, et al. Acupuncture versus ondansetron in the prevention of postoperative vomiting: a study of children undergoing dental surgery. Anaesthesia 2001; 56: 927–32
Yentis SM, Bissonnette B. Ineffectiveness of acupuncture and droperidol in preventing vomiting following strabismus repair in children. Can J Anaesth 1992; 39: 151–4
Ernst E, Pittler MH. Efficacy of ginger for nausea and vomiting: a systematic review of randomized clinical trials. Br J Anaesth 2000; 84: 367–71
Pace JC. Oral ingestion of encapsulated ginger and reported self-care actions for the relief of chemotherapy-associated nausea and vomiting [abstract]. Diss Abstr Int 1987; 47: 3297
Marchioro G, Azzarello G, Viviani F, et al. Hypnosis in the treatment of anticipatory nausea and vomiting in patients receiving cancer chemotherapy. Oncology 2000; 59: 100–4
Morrow GR, Hickok JT. Behavioral treatment of chemotherapy-induced nausea and vomiting. Oncology (Huntingt) 1997; 7: 83–9
Zeltzer L, Kellerman J, Ellenberg L, et al. Hypnosis for reduction of vomiting associated with chemotherapy and disease in adolescents with cancer. J Adolesc Health Care 1983; 4: 77–84
Acknowledgements
No sources of funding were used to assist in the preparation of this manuscript. The authors have no conflicts of interest that are directly relevant to the content of this paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dupuis, L.L., Nathan, P.C. Options for the Prevention and Management of Acute Chemotherapy-Induced Nausea and Vomiting in Children. Pediatr-Drugs 5, 597–613 (2003). https://doi.org/10.2165/00148581-200305090-00003
Published:
Issue Date:
DOI: https://doi.org/10.2165/00148581-200305090-00003