Abstract
Consumers of topical formulations apply a wide spectrum of preparations, both cosmetic and dermatological, to their healthy or diseased skin. These formulations range in physicochemical nature from solid through semisolid to liquid.
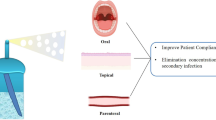
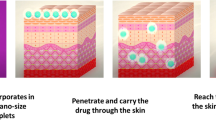
Pharmaceutical foams are pressurized dosage forms containing one or more active ingredients that, upon valve actuation, emit a fine dispersion of liquid and/or solid materials in a gaseous medium. Foam formulations are generally easier to apply, are less dense, and spread more easily than other topical dosage forms. Foams may be formulated in various ways to provide emollient or drying functions to the skin, depending on the formulation constituents. Therefore, this delivery technology should be a useful addition to the spectrum of formulations available for topical use; however, as yet, only a few are commercially available. Probably the most convincing argument for the use of foams is ease of use by the patient, and consumer acceptance. Most foam dosage forms used in dermatology to date have incorporated corticosteroids, although some products have also been used to deliver antiseptics, antifungal agents, anti-inflammatory agents, local anesthetic agents, skin emollients, and protectants.
Although there is no clinical evidence that foam formulations are currently superior to other conventional delivery vehicles, these formulations have a clear application advantage and with continued developments in the science of supersaturation technology, it seems certain that foam delivery systems will retain their place in the dermatological and cosmetic armamentarium.



Similar content being viewed by others
References
Ansel HC, Allen LV, Popovich NG. Pharmaceutical dosage forms and drug delivery systems. 7th ed. Baltimore (MD): Lippincott Williams & Wilkins, 1999
Sciarra JJ, Sciarra CJ. Aerosols. In: Gennaro AR, editor. Remington: the science and practice of pharmacy. 20th ed. Baltimore (MD): Lippincott Williams & Wilkins, 2000: 963–79
Surber C, Smith E. The vehicle: the pharmaceutical carrier of dermatological agents. In: Gabard B, Eisner P, Surber C, et al., editors. Dermato-pharmacology of topical preparations. Berlin: Springer-Verlag, 2000: 5–22
Schwarb FP, Imanidis G, Smith EW, et al. Effect of concentration and degree of saturation of topical fluocinonide formulations on in vitro membrane transport and in vivo availability on human skin. Pharm Res 1999; 16: 909–15
Woodford R, Barry BW. Bioavailability and activity of topical corticosteroids from a novel drug delivery system: the aerosol quick break foam. J Pharm Sci 1977; 66: 99–103
Feldman SR, Sangha N, Setaluri V. Topical corticosteroids in foam vehicle offers comparable coverage compared with traditional vehicles. J Am Acad Dermatol 2000; 42: 1017–20
Feldman SR, Ravis SM, Fleischer Jr AB, et al. Betamethasone valerate in foam vehicle is effective with both daily and twice daily dosing: a single-blind, open-label study in the treatment of scalp psoriasis. J Cutan Med Surg 2001; 5: 386–9
Franz TJ, Parsell DA, Halualani RM, et al. Betamethasone valerate foam 0.12%: a novel vehicle with enhanced delivery and efficacy. Int J Dermatol 1999; 38: 628–32
Melian EB, Spencer CM, Jarvis B. Clobetasol propionate foam 0.05%. Am J Clin Dermatol 2001; 2: 89–92
Cada DJ, Covington TR, Generdli JA, et al., editors. Drug facts and comparisons. St Louis (MO): Facts and Comparisons, 2000: 1597–722
Lebwohl M, Sherer D, Washenik K, et al. A randomized, double-blind, placebo-controlled study of clobetasol propionate 0.05% foam in the treatment of non-scalp psoriasis. Int J Dermatol 2002; 41(5): 269–74
Barditch-Crovo P, Witter F, Hamzeh F, et al. Quantitation of vaginally administered nonoxynol-9 in premenopausal women. Contraception 1997; 55(4): 261–3
Beeuwkes H, Rooij SH. Microbiological tests on operating-theatre staff of a new disinfectant foam based on 1% chlohexidine gluconate. J Hosp Infect 1986; 8(2): 200–2
Arcidiacono R, Zanasi G, Pirone Z, et al. The topical therapy of ulcerative colitis: a multicenter study with beclomethasone dipropionate foam. Minerva Chir 1999; 54(9): 635–44
Ardizzone S, Doldo P, Ranzi T, et al. Mesalazine foam (Salofalk foam) in the treatment of active distal ulcerative colitis: a comparative trial vs Salofalk enema. The SAF-3 study group. Ital J Gastroenterol Hepatol 1999; 31(8): 677–84
Patterson SE, Williams JV, Marks JG. Prevention of sodium lauryl sulphate irritant contact dermatitis by Pro-Q aerosol foam skin protection. J Am Acad Dermatol 1999; 40: 783–5
Pillwein K, Rauschmeier W, Berger H. Clinical experiences with an antimycotic foam preparation with added prednisolone. Padiatr Padol 1986; 21: 75–80
Rekacewicz I, Guillaume JC, Benkhraba F, et al. A double-blind placebo-controlled study of a 2% foaming lotion of ketaconazole in a single application in the treatment of pityriasis versicolor. Ann Dermatol Venereol 1990; 117: 709–11
Daffonchio L, Bestetti A, Clavenna G, et al. Effects of a new foam formulation of ketoprofen lysine salt in experimental models of inflammation and hyperalgesia. Arzneimittel Forschung 1995; 45(5): 590–4
Parrini M, Cabitza P, Arrigo A, et al. Efficacy and tolerability of ketoprofen lysine salt foam for topical use in the treatment of traumatic pathologies of the locomotor apparatus. Clin Ther 1992; 141(9): 199–204
Müller M, Rastelli C, Ferri P, et al. Transdermal penetration of diclofenac after multiple epicutaneous administration. J Rheumatol 1998; 25(9): 1833–6
Purdon CH. In vitro passage of ibuprofen through synthetic and biological membranes [thesis]. Grahamstown: Rhodes University, 2002
Acknowledgements
No sources of funding were used to assist in the preparation of this manuscript. The authors have no conflicts of interest that are directly relevant to the content of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Purdon, C.H., Haigh, J.M., Surber, C. et al. Foam Drug Delivery in Dermatology. Am J Drug Deliv 1, 71–75 (2003). https://doi.org/10.2165/00137696-200301010-00006
Published:
Issue Date:
DOI: https://doi.org/10.2165/00137696-200301010-00006