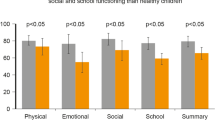
Abstract
Recurrent respiratory tract infections are common in children. They reflect the immaturity of the immune system in its encounter with environmental antigens. Little or no specific protective immune response has yet been established. These infections represent an important public health problem in terms of both treatment (anti-inflammatory or antibacterial drugs for children) and economy.
Immunotherapy has been proposed as a means of preventing these recurrent infections by providing children with small doses of inactive bacterial antigens liable to trigger specific and protective immune responses. Among such drugs, ribosomal preparations (to which this review is limited) appear to be not only well tolerated, but also ideally targeted to induce mucosal responses. One preparation of ribosomal mucosal vaccine is commercially available in several countries. Numerous clinical trials in the world have confirmed the positive role of this mucosal ribosomal bacterial vaccine in significantly reducing the number of infections, courses of antibacterials, and absenteeism. In vitro and ex vivo investigations have confirmed that such vaccines indeed trigger protective specific immune responses.
Similar content being viewed by others
References
Fendrick AM, Saint S, Brook I, et al. Diagnosis and treatment of upper respiratory tract infections in the primary care setting. Clin Ther 2001; 23: 1683–706
Peat JK, Keena V, Harakeh Z, et al. Parental smoking and respiratory tract infections in children. Paediatr Respir Rev 2001; 2: 207–13
Mogyoros M. Challenges of managed care organizations in treating respiratory tract infections in an age of antibiotic resistance. Am J Manag Care 2001; 7(6 Suppl.): S163–169
MacDonald TT, Spencer J. Ontogeny of the mucosal immune response. Springer Semin Immunopathol 1990; 12: 129–37
Hanson LA, Ceafalau L, Mattsby-Baltzer I, et al. The mammary gland-infant intestine immunologic dyad. Adv Exp Med Biol 2000; 478: 65–76
Ritchie RF, Palomaki GE, Neveux LM, et al. Reference distributions for immunoglobulins A, G, and M: a practical, simple, and clinically relevant approach in a large cohort. J Clin Lab Anal 1998; 12: 363–70
Gorfien JL, Hard R, Noble B, et al. Quantitative study of germinal center area in normal and diseased tonsils using image analysis. Ann Otol Rhinol Laryngol 1999; 108: 398–402
Kyd JM, Cripps AW. Killed whole bacterial cells, a mucosal delivery system for the induction of immunity in the respiratory tract and middle ear: an overview. Vaccine 1999; 17: 1775–81
Pace JL, Rossi HA, Esposito VM, et al. Inactivated whole-cell bacterial vaccines: current status and novel strategies. Vaccine 1998; 16: 1563–74
Derenne JP, Delclaux B. Clinical experience with OM-85 BV in upper and lower respiratory tract infections. Respiration 1992; 59Suppl. 3: 28–31
Youmans AS, Youmans GP. Immunogenic activity of a ribosomal fraction obtained from Mycobacterium tuberculosis. J Bacteriol 1965; 89: 1291–8
Gregory RL. Microbial ribosomal vaccines. Rev Infect Dis 1986; 8: 208–17
Fujimura Y. Evidence of M-cells as portals of entry for antigens in the nasopharyngeal lymphoid tissue of humans. Virchows Arch 2000; 436: 560–6
Neutra MR. Current concepts in mucosal immunity: V role of M cells in transepithelial transport of antigens and pathogens to the mucosal immune system. Am J Physiol 1998; 274: G785–791
Kraal G, Mebius RE. High endothelial venules: lymphocyte traffic control and controlled traffic. Adv Immunol 1997; 65: 347–95
Caliot E, Libon C, Kernels S, et al. Translocation of ribosomal immunostimulant through an in vitro-reconstituted digestive barrier containing M-like cells. Scand J Immunol 2000; 52: 588–94
Béné MC, Zanin C, Perrin P, et al. Specific antibody-producing cells in humans after oral immunization with a ribosomal vaccine Ribomunyl. Adv Exp Med Biol. 1995; 371B: 1563–6
Zanin C, Perrin P, Béné MC, et al. Antibody-producing cells in peripheral blood and tonsils after oral treatment of children with bacterial ribosomes. Int J Immunopharmacol 1994; 16: 497–505
Kolopp-Sarda MN, Béné ME, Allaire JM, et al. Kinetics of specific salivary IgA responses in man after oral challenge by ribosomal immunostimulant. Int J Immunopharmacol 1997; 19: 181–6
Hbabi-Haddioui L, Roques C. Inhibition of Streptococcus pneumoniae adhesion by specific salivary IgA after oral immunisation with a ribosomal immunostimulant. Drugs 1997; 54Suppl. 1: 29–32
Michel FB, Dussourd d’Hinterland L, Pinel AM, et al. Prevention of bacterial respiratory infection by an association of bacterial ribosomes and membranous proteoglycans (II): objective assessment of the response and of the immunological stimulation. Arzneimittel Forschung 1980; 30: 198–206
Girard JP, Gumowski PI, Chavaz A, et al. Rôle de différentes formes de Ribomunyl dans les infections respiratoires récidivantes. Schweiz Med Wochenschr 1988; 118: 856–61
Fiocchi A, Vignati B, Cinquepalmi P, et al. Treatment using immucytal in children with recurrent respiratory infections: an Italian multicenter experience. Pediatr Med Chir 1989; 11: 285–91
Fiocchi A, Arancio R, Cinquepalmi P, et al. Recurrent respiratory infections in childhood: experience with a bacterial extract plus bacterial ribosomes (Immucytal). J Int Med Res 1990; 18: 50–60
Venturi MC, Biolchini A, Bardare M. Local immunotherapy prevention of recurrent respiratory infections in children. Minerva Pediatr 1991; 43: 557–61
Gordienko SM, Lasitsa OI, Misharina ZA, et al. The correction of the immune system by using the immunomodulator ribomunyl in patients with recurrent respiratory tract infections [in Russian]. Ter Arkh 1995; 67: 32–8
Mora R, Barbieri M, Passali GC, et al. A preventive measure for otitis media in children with upper respiratory tract infections. Int J Pediatr Otorhinolaryngol 2002; 63: 111–8
Serrano E, Demanez JP, Morgon A, et al. Effectiveness of ribosomal fractions of Klebsiella pneumoniae, Streptococcus pneumoniae, Streptococcus pyogenes, Haemophilus influenzae and the membrane fraction of Kp (Ribomunyl®) in the prevention of clinical recurrences of infectious rhinitis: results of a multicenter double-blind placebo-controlled study. Eur Arch Otorhinolaryngol 1997; 254: 372–5
Boyle P, Bellanti JA, Robertson C. Meta-analysis of published clinical trials of a ribosomal vaccine (Ribomunyl®) in prevention of respiratory infections. Biodrugs 2000; 14: 389–408
Simecka JW. Mucosal immunity of the gastrointestinal tract and oral tolerance. Adv Drug Deliv Rev 1998; 34: 235–59
BIAM (Bank of Data Automated on the Drugs), Paris. Ribomunyl granules pour solution buvable en sachet [online]. Available from URL: http://www.biam2.org/www/Spe5374.html [Accessed 2002 Sept 11]
Clot J. Pharmacology of ribosomal immunotherapy. Drugs 1997; 54Suppl. 1: 33–6
Banz K, Schwicker D, Thomas AM. Economic evaluation of immunoprophylaxis in children with recurrent ear, nose and throat infections. Pharmacoeconomics 1994; 6: 464–77
Acknowledgements
The authors are grateful to Drs Gérard Normier, Anne Marie Perruchet and Anne Marie Thomas for fruitful collaboration on the topic of ribosomal immunotherapy.
No sources of funding were used to assist in the preparation of this manuscript. The authors have no conflicts of interest that are directly relevant to the content of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Béné, M.C., Faure, G.C. Ribosomal Immunotherapy for Recurrent Respiratory Tract Infections in Children. Pediatr-Drugs 5, 223–228 (2003). https://doi.org/10.2165/00128072-200305040-00002
Published:
Issue Date:
DOI: https://doi.org/10.2165/00128072-200305040-00002