Abstract
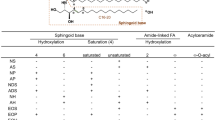
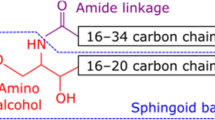
Stratum corneum intercellular lipids play an important role in the regulation of skin water barrier homeostasis and water-holding capacity. Modification of intercellular lipid organization and composition may impair these properties. Patients with skin diseases such as atopic dermatitis, psoriasis, contact dermatitis, and some genetic disorders have diminished skin barrier function. Lipid composition in diseased skin is characterized by decreased levels of ceramide and altered ceramide profiles. To clarify mechanisms underlying ceramides as a causative factor of skin disease, investigators have examined the activity of enzymes in the stratum corneum on ceramide production and degradation. The activities of ceramidase, sphingomyelin deacylase, and glucosylceramide deacylase are increased in epidermal atopic dermatitis. Investigators have also compared the expression levels of sphingolipid activator protein in the epidermis of normal and diseased skin. A decreased level of prosaposin has been identified in both atopic dermatitis and psoriasis. These results indicate that decreased ceramide level is a major etiologic factor in skin diseases. Hence, topical skin lipid supplementation may provide opportunities for controlling ceramide deficiency and improving skin condition.



Similar content being viewed by others
References
Bouwstra JA, Honeywell-Nguyen PL, Gooris GS, et al. Structure of the skin barrier and its modulation by vesicular formulations. Prog Lipid Res. 2003; 42: 1–36
Imokawa G, Abe A, Jin K, et al. Decreased level of ceramides in stratum comeum of atopic dermatitis: an etiologic factor in atopic dry skin. J Invest Dermatol. 1991; 96: 523–6
Paige DG, Morse-Fisher N, Harper JI. Quantification of stratum comeum ceramides and lipid envelope ceramides in the hereditary ichthyoses. Br J Dermalot. 1994; 131: 23–7
Matsumoto M, Umemoto N, Sugiura H, et al. Difference in ceramide composition between “dry” and “normal” skin in patients with atopic dermatitis. Acta Derm Venereol. 1999; 79: 246–7
Pilgram GS, Vissers DC, van der Meulen H, et al. Aberrant lipid organization in stratum corneum of patients with atopic dermatitis and lamellar ichthyosis. J Invest Dermatol. 2001; 117: 710–7
Macheleidt O, Kaiser HW, Sandhoff K. Deficiency of epidermal protein-bound omega-hydroxyceramides in atopic dermatitis. J Invest Dermatol. 2002; 119: 166–73
Geilen CC, Wieder T, Orfanos CE. Ceramide signalling: regulatory role in cell proliferation, differentiation and apoptosis in human epidermis. Arch Dermatol Res. 1997; 289 (10): 559–66
Burek C, Roth J, Koch HG, et al. The role of ceramide in receptor and stress-induced apoptosis studied in acidic ceramidase-deficient Farber disease cells. Oncogene. 2001; 20: 6493–502
Komatsu M, Takahashi T, Abe T, et al. Evidence for the association of ultraviolet-C and Hb2Ob2-induced apoptosis with acid sphingomyelinase activation. Biochim Biophys Acta. 2001; 1533: 47–54
Separovic D, Mann KJ, Oleinick NL. Association of ceramide accumulation with photodynamic treatment-induced cell death. Photochem Photobiol 1998; 68: 101–9
Pena LA, Fuks Z, Kolesnick R. Stress-induced apoptosis and the sphingomyelin pathway. Biochem Pharmacol. 1997; 53: 615–21
Kolesnick R. The therapeutic potential of modulating the ceramide/sphingomyelin pathway. J Clin Invest. 2002; 110: 3–8
Ogretmen B, Hannun YA. Updates on functions of ceramides in chemotherapy-induced cell death and in multidrug resistance. Drug Resist Updat. 2001; 4: 368–77
Claus R, Russwurm S, Meisner M, et al. Modulation of the ceramide level: a novel therapeutic concept?. Curr Drug Targets. 2000; 1: 185–205
Lucci A, Han TY, Liu YY, et al. Multidrug resistance modulators and doxorubicin synergize to elevate ceramide levels and elicit apoptosis in drug-resistant cancer cells. Cancer. 1999; 86: 300–11
Charles AG, Han TY, Liu YY, et al. Taxol-induced ceramide generation and apoptosis in human breast cancer cells. Cancer Chemother Pharmaco. 2001; 47: 444–50
Holleran WM, Feingold KR, Man MQ, et al. Regulation of epidermal sphingolipid synthesis by permeability barrier function. J Lipid Res. 1991; 32 (7): 1151–8
Holleran WM, Takagi Y, Menon GK, et al. Permeability barrier requirements regulate epidermal β-glucocerebrosidase. J Lipid Res. 1995; 35: 905–12
Jensen JM, Schiitze S, Förl M, et al. Roles for tumor necrosis factor receptor p55 and sphingomyelinase in repairing the cutaneous permeability barrier. J Clin Invest. 1999; 104: 1761–70
Higuchi K, Hara J, Okamoto R, et al. The skin of atopic dermatitis patients contains a novel enzyme, glucosylceramide sphingomyelin deacylase, which cleaves the N-acyl linkage of sphingomyelin and glucosylceramide. Biochem J. 2000; 350: 747–56
Hara J, Higuchi K, Okamoto R, et al. High-expression of sphingomyelinase deacylase is an important determinant of ceramide deficiency leading to barrier disruption atopic dermatitis. J Invest Dermatol. 2000; 115: 406–13
Ishibashi M, Arikawa J, Okamoto R, et al. Abnormal expression of the novel epidermal enzyme, glucosylceramide deacylase, and the accumulation of its enzymatic reaction product, glucosylsphingosine, in the skin of patients with atopic dermatitis. Lab Invest. 2003; 83: 397–408
Wilkening G, Linke T, Sandhoff K. Lysosomal degradation on vesicular membrane surfaces. J Biol Chem. 1998; 273: 30271–8
Tayama M, Soeda S, Kishimoto Y, et al. Effect of saposins on acid sphingomyelinase. Biochem J. 1993; 290: 401–4
Hammond SA, Tsonis C, Sellins K, et al. Transcutaneous immunization of domestic animals: opportunities and challenges. Adv Drug Deliv Rev. 2000; 43: 45–55
Ponec M, Weerheim A, Lankhorst P, et al. New acylceramide in native and reconstructed epidermis. J Invest Dermatol. 2003; 120: 581–8
Bouwstra JA, Gooris GS, Dubbelaar FER, et al. Phase behavior of stratum corneum lipid mixtures based on human ceramides: the role of natural and synthetic ceramide 1. J Invest Dermatol. 2002; 118: 606–17
Bouwstra JA, Gooris GS, Dubbelaar FER, et al. Phase behavior of lipid mixtures based on human ceramides: coexistence of crystalline and lipid phases. J Lipid Res. 2001; 42: 1759–70
White SH, Mirejovsky D, King GI. Structure of lamellar lipid domains and corneocyte envelopes of murine stratum corneum: an x-ray diffraction study. Biochemistry. 1988; 27: 3725–32
Bouwstra JA, Goods GS, van der Spek JA, et al. Structural investigations of human stratum comeum by small-angle x-ray scattering. J Invest Dermatol. 1991; 97: 1005–12
Bouwstra JA, Goods GS, van der Spek JA, et al. The lipid and protein structure of mouse stratum comeum: a wide and small angle diffraction study. Biochim Biophys Acta. 1994; 1212: 183–92
Pilgram GS, Engelsma-van Pelt AM, Bouwstra JA, et al. Electron diffraction provides new information on human stratum comeum lipid organization studied in relation to death and temperature. J Invest Dermatol. 1999; 113: 403–9
Bouwstra JA, Honeywell-Nguyen PL. Skin structure and mode of vesicles. Adv Drug Deliv Rev. 2002; 54: s41–55
Jin K, Higaki Y, Tagaki Y, et al. Analysis of beta-glucocerebrosidase and ceramidase activities in atopic and aged dry skin. Acta Derm Venereol. 1994; 74: 337–40
Imokawa G, Akasaki S, Hattori M, et al. Selective recovery of deranged water-holding properties by stratum lipids. J Invest Dermatol. 1986; 87: 758–61
Di Nardo A, Wertz P, Giannetti A, et al. Ceramide and cholesterol composition of the skin of patients with atopic dermatitis. Acta Derm Venereol. 1998; 78: 27–30
Murata Y, Ogata J, Higaki Y, et al. Abnormal expression of sphingomyelin acylase in atopic dermatitis: an etiologic factor for ceramide deficiency?. J Invest Dermatol. 1996; 106: 1242–9
Yamamoto A, Serizawa S, Ito M, et al. Stratum comeum lipid abnormalities in atopic dermatitis. Arch Dermatol Res. 1991; 283: 219–23
Ohnishi Y, Okino N, Ito M, et al. Ceramidase activity in bacterial skin flora as a possible cause of ceramide deficiency in atopic dermatitis. Clin Diagn Lab Immunol. 1999; 6: 101–4
Kusuda S, Chang-Yi C, Takahashi M, et al. Localization of sphingomyelinase in lesional skin of atopic dermatitis patients. J Invest Dermatol. 1998; 111: 733–8
Chang-Yi C, Kusuda S, Seguchi T, et al. Decreased level of prosaposin in atopic skin. J Invest Dermatol. 1997; 109: 319–23
Jensen JM, Folster-Holst R, Baranowsky A, et al. Impaired sphingomyelinase activity and epidermal differentiation in atopic dermatitis. J Invest Dermatol. 2004; 122 (6): 1423–31
Arikawa J, Ishibashi M, Kawashima M, et al. Decreased levels of sphingosine, a natural antimicrobial agent, may be associated with vulnerability of the stratum comeum from patients with atopic dermatitis to colonization by Staphylococcus aureus. J Invest Dermatol. 2002; 119: 433–9
Okamoto R, Arikawa J, Ishibashi M, et al. Sphingosylphosphorylcholine is upregulated in the stratum corneum of patients with atopic dermatitis. J Lipid Res. 2003; 44: 93–102
Uchida Y, Hara M, Nishio M, et al. Epidermal sphingomyelins are precursors for selected stratum comeum ceramides. J Lipid Res. 2000; 41: 2071–82
Motta S, Monti M, Sesana S, et al. Abnormality of water barrier function in psoriasis: role of ceramide functions. Arch Dermatol. 1994; 130: 452–6
Motta S, Sesana S, Monti M, et al. Interlamellar lipid difference between normal and psoriatic stratum corneum. Acta Derm Venereol Suppl. 1994; 186: 131–2
Motta S, Monti M, Sesana S, et al. Ceramide composition of the psoriatic scale. Biochim Biophys Acta. 1993; 1182: 147–51
Alessandrini F, Stachowitz S, Ring J, et al. The level of prosaposin is decreased in the skin of patients with psoriasis vulgaris. J Invest Dermatol. 2001; 116: 394–400
Sidransky E, Fartasch M, Lee RE, et al. Epidermal abnormalities may distinguish type 2 from type 1 and type 3 of Gaucher disease. Pediatr Res. 1996; 39: 134–41
Holleran WM, Ginns EI, Menon GK, et al. Consequences of β-glucocerebrosidase deficiency in epidermis: ultrastructure and permeability barrier alterations in Gaucher disease. J Clin Invest. 1994; 93 (4): 1756–64
Doering T, Proia RL, Sandhoff K. Accumulation of protein-bound epidermal glucosylceramides in β-glucocerebrosidase deficient type 2 Gaucher mice. FEBS Lett. 1999; 447: 167–70
Orbisky E, Sidransky E, McKinney CE, et al. Glucosylsphingosine accumulation in mice and patients with type 2 Gaucher disease begins early in gestation. Pediatr Res. 2000; 48: 233–7
Ida H, Rennert OM, Eto Y, et al. Cloning of a human acid sphingomyelinase cDNA with a new mutation that renders the enzyme inactive. J Biochem. 1993; 114: 15–20
Schmuth M, Man MQ, Weber F, et al. Permeability barrier disorder in Niemann-Pick disease: sphingomyelin-ceramide processing required for normal barrier homeostasis. J Invest Dermatol. 2000; 115: 459–66
Muramatsu T, Sakai N, Yanagihara I, et al. Mutation analysis of the acid ceramidase gene in Japanese patients with Farber disease. J Inherit Metab Dis. 2002; 25: 585–92
De Paepe K, Roseeuw D, Rogiers V. Repair of acetone-and sodium lauryl sulfate-damaged human skin barrier function using topically applied emulsions containing barrier lipids. J Fur Acad Dermatol Venereol. 2002; 16: 587–94
Coderch L, De Pera M, Fonollosa J, et al. Efficacy of stratum comeum lipid supplementation on human skin. Contact Dermatitis. 2002; 47: 139–46
Man MM, Feingold KR, Thomfeldt CR, et al. Optimization of physiological lipid mixtures for barrier repair. J Invest Dermatol. 1996; 106: 1096–101
Berardesca E, Barbareschi M, Veraldi S, et al. Evaluation of efficiency of a skin lipid mixture in patients with irritant contact dermatitis, allergic contact dermatitis or atopic dermatitis: a multicenter study. Contact Dermatitis. 2001; 45: 280–5
Kucharekova M, Schalkwijk J, van de Kerkhof PCM, et al. Effect of a lipid-rich emollient containing ceramide 3 in experimentally induced skin barrier dysfunction. Contact Dermatitis. 2002; 46: 331–8
Chamlin SL, Kao J, Frieden IJ, et al. Ceramide-dominant barrier repair lipids alleviate childhood atopic dermatitis: changes in barrier function provide a sensitive indicator of disease activity. J Am Acad Dermatol. 2002; 47: 198–208
Acknowledgments
The authors have provided no information on sources of funding or on conflicts of interest directly relevant to the content of this review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Choi, M.J., Maibach, H.I. Role of Ceramides in Barrier Function of Healthy and Diseased Skin. Am J Clin Dermatol 6, 215–223 (2005). https://doi.org/10.2165/00128071-200506040-00002
Published:
Issue Date:
DOI: https://doi.org/10.2165/00128071-200506040-00002