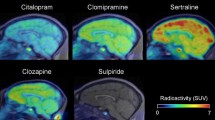
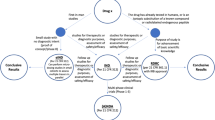
Abstract
Positron emission tomography (PET)-microdosing comprises the administration of a carbon-11- or fluorine-18-labelled drug candidate to human subjects in order to describe the drug’s concentration-time profile in body tissues targeted for treatment. As PET microdosing involves the administration of only microgram amounts of unlabelled drug, the potential toxicological risk to human subjects is very limited. Consequently, regulatory authorities require reduced preclinical safety testing as compared with conventional phase 1 studies. Microdose studies are gaining increasing importance in clinical drug research as they have the potential to shorten time-lines and cut costs along the critical path of drug development. Current applications of PET in anticancer, anti-infective and CNS system drug research are reviewed.

Similar content being viewed by others
References
DiMasi JA. Risks in new drug development: approval success rates for investigational drugs. Clin Pharmacol Ther 2001; 69 (5): 297–307
Kola I, Landis J. Can the pharmaceutical industry reduce attrition rates? Nat Rev Drug Discov 2004; 3 (8): 711–5
Tollman P, Guy P, Altshuler J, et al. A revolution in R&D: how genomics and genetics are transforming the biopharmaceutical industry. Boston Consulting Group Report 2001: 1–64
DiMasi JA, Hansen RW, Grabowski HG. The price of innovation: new estimates of drug development costs. J Health Econ 2003; 22 (2): 151–85
DiMasi JA, Grabowski HG. Economics of new oncology drug development. J Clin Oncol 2007; 25 (2): 209–16
Goodall S, Ringel M, Tollman P. Rising to the productivity challenge: a strategic framework for Biopharma. Boston Consulting Group Report 2004: 1–12
Sarapa N. Early human microdosing to reduce attrition in clinical drug development. Am Pharm Outsourcing 2003; 4 (5): 42–7
European Medicines Agency. Committee for Medicinal Products for Human Use (CHMP) position paper on non-clinical safety studies to support clinical trials with a single microdose. CPMP/SWP/2599/02 [online]. Available from URL: http://www.emea.eu.int/pdfs/human/swp/259902en.pdf [Accessed 2008 Jan 20]
US Food and Drug Administration. Center for Drug Evaluation and Research (CDER) guidance for industry, investigators, and reviewers: exploratory IND studies [online]. Available from URL: http://www.fda.gov/cder/guidance/7086fnl.htm [Accessed 2008 Jan 20]
Lappin G, Kuhnz W, Jochemsen R, et al. Use of microdosing to predict pharmacokinetics at the therapeutic dose: experience with 5 drugs. Clin Pharmacol Ther 2006; 80 (3): 203–15
European Union Microdose AMS Partnership Programme [online]. Available from URL: http://www.eumapp.com [Accessed 2008 Jan 31]
Zanni GR, Wick JY. Microdosing: the new pharmacokinetic paradigm. Consult Pharm 2006; 21 (10): 756–76
Buchan P. Smarter candidate selection: utilizing microdosing in exploratory clinical studies. Ernst Schering Res Found Workshop 2007; 59: 7–27
Wagner CC, Müller M, Lappin G, et al. Positron emission tomography for use in microdosing studies. Curr Opin Drug Discov Devel 2008; 11 (1): 104-10
Liu P, Muller M, Derendorf H. Rational dosing of antibiotics: the use of plasma concentrations versus tissue concentrations. Int J Antimicrob Agents 2002; 19 (4): 285–90
Meibohm B, Derendorf H. Basic concepts of pharmacokinetic/pharmacodynamic (PK/PD) modelling. Int J Clin Pharmacol Ther 1997; 35 (10): 401–13
Müller M, de la Peña A, Derendorf H. Issues in pharmacokinetics and pharmacodynamics of anti-infective agents: II. Tissue distribution. Antimicrob Agents Chemother 2004; 48 (5): 1441–53
Bertino Jr JS, Greenberg HE, Reed MD. American College of Clinical Pharmacology position statement on the use of microdosing in the drug development process. J Clin Pharmacol 2007; 47 (4): 418–22
Garner RC. Less is more: the human microdosing concept. Drug Discov Today 2005; 10 (7): 449–51
Garner RC, Goris I, Laenen AA, et al. Evaluation of accelerator mass spectrometry in a human mass balance and pharmacokinetic study-experience with 14C-labeled (R)-6-[amino(4-chlorophenyl)(1-methyl-1H-imidazol-5-yl)methyl]-4-(3-chlorophenyl)-1-methyl-2(1H) quinolinone (R115777), a farnesyl transferase inhibitor. Drug Metab Dispos 2002; 30 (7): 823–30
Lappin G, Garner R. A review of human phase 0 (microdosing) clinical trials following the US Food and Drug Administration exploratory investigational new drug studies guidance. Int J Pharm Med 2006; 20 (3): 159–65
Lappin G, Garner RC. Big physics, small doses: the use of AMS and PET in human microdosing of development drugs. Nat Rev Drug Discov 2003; 2 (3): 233–40
Balani SK, Nagaraja NV, Qian MG, et al. Evaluation of microdosing to assess pharmacokinetic linearity in rats using liquid chromatography-tandem mass spectrometry. Drug Metab Dispos 2006; 34 (3): 384–8
Elsinga PH. Radiopharmaceutical chemistry for positron emission tomography. Methods 2002; 27 (3): 208–17
Saleem A, Aboagye EO, Matthews JC, et al. Plasma pharmacokinetic evaluation of cytotoxic agents radiolabelled with positron emitting radioisotopes. Cancer Chemother Pharmacol. Epub 2007
Lundqvist H, Antoni G, Langstrom B. Genotoxic hazard of radiopharmaceuticals in humans: chemical and radiation aspects coupled to microdosing. Eur J Clin Pharmacol 2007; 63 (7): 641–5
Combes RD, Berridge T, Connelly J, et al. Early microdose drug studies in human volunteers can minimise animal testing. Proceedings of a workshop organised by Volunteers in Research and Testing. Eur J Pharm Sci 2003; 19 (1): 1–11
Lappin G, Rowland M, Garner RC. The use of isotopes in the determination of absolute bioavailability of drugs in humans. Expert Opin Drug Metab Toxicol 2006; 2 (3): 419–27
Chiou WL, Lam G, Chen ML, et al. Arterial-venous plasma concentration differences of six drugs in the dog and rabbit after intravenous administration. Res Commun Chem Pathol Pharmacol 1981; 32 (1): 27–39
Sandhu P, Vogel JS, Rose MJ, et al. Evaluation of microdosing strategies for studies in preclinical drug development: demonstration of linear pharmacokinetics in dogs of a nucleoside analog over a 50-fold dose range. Drug Metab Dispos 2004; 32 (11): 1254–9
Fischman AJ, Alpert NM, Rubin RH. Pharmacokinetic imaging: a noninvasive method for determining drug distribution and action. Clin Pharmacokinet 2002; 41 (8): 581–602
Wolf W. Imaging can be much more than pretty pictures. Pharm Res 1995; 12 (12): 1821–2
Bergström M, Grahnen A, Langström B. Positron emission tomography microdosing: a new concept with application in tracer and early clinical drug development. Eur J Clin Pharmacol 2003; 59 (5–6): 357–66
Halldin C, Gulyas B, Farde L. PET studies with carbon-11 radioligands in neuropsychopharmacological drug development. Curr Pharm Res 2001; 7 (18): 1907–29
Hietala J. Ligand-receptor interactions as studied by PET: implications for drug development. Ann Med 1999; 31 (6): 438–43
Tauscher J, Kapur S. Choosing the right dose of antipsychotics in schizophrenia: lessons from neuroimaging studies. CNS Drugs 2001; 15 (9): 671–8
Kasper S, Tauscher J, Willeit M, et al. Receptor and transporter imaging studies in schizophrenia, depression, bulimia and Tourette’s disorder: implications for psychopharmacology. World J Biol Psychiatry 2002; 3 (3): 133–46
Andree B, Hedman A, Thorberg SO, et al. Positron emission tomographic analysis of dose-dependent NAD-299 binding to 5-hydroxytryptamine-1A receptors in the human brain. Psychopharmacology (Berl) 2003; 167 (1): 37–45
de Vries EF, Rots MG, Hospers GA. Nuclear imaging of hormonal receptor status in breast cancer: a tool for guiding endocrine treatment and drug development. Curr Cancer Drug Targets 2007; 7 (6): 510–9
Bergström M, Hargreaves JR, Burns HD, et al. Human positron emission tomography studies of brain neurokinin 1 receptor occupancy by aprepitant. Biol Psychiatry 2004; 55 (10): 1007–12
Dimitrakopoulou A, Strauss LG, Clorius JH, et al. Studies with positron emission tomography after systemic administration of fluorine-18-uracil in patients with liver metastases from colo-rectal carcinoma. J Nucl Med 1993; 34 (7): 1075–81
Harte RJ, Matthews JC, O’sReilly SM, et al. Tumor, normal tissue, and plasma pharmacokinetic studies of fluorouracil biomodulation with N-phosphonacetyl-L-aspartate, folinic acid, and interferon alfa. J Clin Oncol 1999; 17 (5): 1580–8
Saleem A, Yap J, Osman S, et al. Modulation of fluorouracil tissue pharmacokinetics by eniluracil: in-vivo imaging of drug action. Lancet 2000; 355 (9221): 2125–31
Aboagye EO, Saleem A, Cunningham VJ, et al. Extraction of 5-fluorouracil by tumor and liver: a noninvasive positron emission tomography study of patients with gastrointestinal cancer. Cancer Res 2001; 61 (13): 4937–41
Saleem A, Harte RJ, Matthews JC, et al. Pharmacokinetic evaluation of N-[2-(dimethylamino)ethyl]acridine-4-carbox-amide in patients by positron emission tomography. J Clin Oncol 2001; 19 (5): 1421–9
Hill TP. Phase 0 trials: are they ethically challenged? Clin Cancer Res 2007; 13 (3): 783–4
Marchetti S, Schellens JH. The impact of FDA and EMEA guidelines on drug development in relation to phase 0 trials. Br J Cancer 2007; 97 (5): 577–81
Fischman AJ, Alpert NM, Livni E, et al. Pharmacokinetics of 18F-labeled fluconazole in healthy human subjects by positron emission tomography. Antimicrob Agents Chemother 1993; 37 (6): 1270–7
Tewson TJ. Labeled antibiotics: positron emission tomography as a tool for measuring tissue distribution. Drug Dev Res 2003; 59: 261–8
Müller M. Science, medicine, and the future: microdialysis. BMJ 2002; 324 (7337): 588–91
Fischman AJ, Babich JW, Bonab AA, et al. Pharmacokinetics of [18F]trovafloxacin in healthy human subjects studied with positron emission tomography. Antimicrob Agents Chemother 1998; 42 (8): 2048–54
Brunner M, Langer O, Dobrozemsky G, et al. [18F]Ciprofloxacin, a new positron emission tomography tracer for noninvasive assessment of the tissue distribution and pharmacokinetics of ciprofloxacin in humans. Antimicrob Agents Chemother 2004; 48 (10): 3850–7
Langer O, Karch R, Müller U, et al. Combined PET and microdialysis for in vivo assessment of intracellular drug pharmacokinetics in humans. J Nucl Med 2005; 46(11): 1835–41.
Hyatt JM, McKinnon PS, Zimmer GS, et al. The importance of pharmacokinetic/pharmacodynamic surrogate markers to outcome. Focus on antibacterial agents. Clin Pharmacokin 1995; 28 (2): 143–60
Gau W, Kurz J, Petersen U, et al. Isolation and structural elucidation of urinary metabolites of ciprofloxacin. Arzneimittelforschung 1986; 36 (10): 1545–9
Babich JW, Rubin RH, Graham WA, et al. 18F-Labeling and biodistribution of the novel fluoro-quinolone antimicrobial agent, trovafloxacin (CP 99,219). Nucl Med Biol 1996; 23 (8): 995–8
Tewson TJ, Yang D, Wong G, et al. The synthesis of fluorine-18 lomefloxacin and its preliminary use in human studies. Nucl Med Biol 1996; 23 (6): 767–72
Fischman AJ, Alpert NM, Babich JW, et al. The role of positron emission tomography in pharmacokinetic analysis. Drug Metabol Rev 1997; 29 (4): 923–56
Salazar DE, Fischman AJ. Central nervous system pharmaco-kinetics of psychiatric drugs. J Clin Pharmacol 1999 Suppl.: 10S–12S
Farde L. The advantage of using positron emission tomography in drug research. Trends Neurosciences 1996; 19 (6): 211–4
Bauer M, Langer O, Dal-Bianco P, et al. A positron emission tomography microdosing study with a potential antiamyloid drug in healthy volunteers and patients with Alzheimer’s disease. Clin Pharmacol Ther 2006; 80 (3): 216–27
Langer O, Krcal A, Schmid A, et al. Synthesis of 1,1 ′[C-11]-methylene-di-(2-naphthol) ([C-11]ST1859) for PET studies in humans. J Labelled Compounds Radiopharm 2005; 48 (8): 577–87
Angelini G. Simplified synthesis of 1,1 ′[C-14]-methylene-di (2-naphthol): a radiochemical and kinetic approach. J Labelled Compounds Radiopharm 2004; 47 (9): 543–56
Acknowledgements
No sources of funding were used to assist in the preparation of this review. The authors have no conflicts of interest that are directly relevant to the content of this review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bauer, M., Wagner, C.C. & Langer, O. Microdosing Studies in Humans. Drugs R&D 9, 73–81 (2008). https://doi.org/10.2165/00126839-200809020-00002
Published:
Issue Date:
DOI: https://doi.org/10.2165/00126839-200809020-00002