Abstract
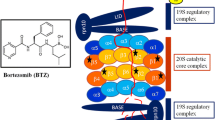
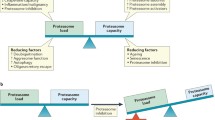
The majority of intracellular proteins undergo degradation through the ubiquitin-proteasome pathway. The proteasome pathway has a role in regulating cell proliferation, differentiation, survival and apoptosis. The naturally occurring proteasome inhibitor lactacystin was the first proteasome inhibitor noted to induce apoptosis in vitro. Compared with first-generation proteasome inhibitors, bortezomib (PS-341), a dipeptide boronic acid, has exhibited higher potency and specificity, and has been approved for the treatment of relapsed or refractory myeloma. However, there are some patients who do not respond to therapy or who respond briefly and then relapse. It is becoming increasingly clear that myeloma cells respond to the stress caused by proteasome inhibitors (bortezomib) via rapidly up-regulating pathways that suppress apoptosis, thus attenuating its antitumour activity. The delineation of these molecular pathways and mechanisms to circumvent them are needed to allow this important class of agents to remain vital in the armamentarium of the management of multiple myeloma and other malignancies.


Similar content being viewed by others
References
Orlowski RZ. The role of the ubiquitin-proteasome pathway in apoptosis. Cell Death Differ 1999; 6: 303–13
Baumeister W, Walz J, Zuhl F, et al. The proteasome: paradigm of a self-compartmentalizing protease. Cell 1998; 92: 367–80
Kisselev AF, Goldberg AL. Proteasome inhibitors: from research tools to drug candidates. Chem Biol 2001; 8: 739–58
Hershko A. Roles of ubiquitin-mediated proteolysis in cell cycle control. Curr Opin Cell Biol 1997; 9: 788–99
Imajoh-Ohmi S, Kawaguchi T, Sugiyama S, et al. Lactacystin, a specific inhibitor of the proteasome, induces apoptosis in human monoblast U937 cells. Biochem Biophys Res Commun 1995; 217: 1070–7
Voorhees PM, Dees EC, O’Neil B, et al. The proteasome as a target for cancer therapy. Clin Cancer Res 2003; 9: 6316–25
Fujita E, Mukasa T, Tsukahara T, et al. Enhancement of CPP32-like activity in the TNF-treated U937 cells by the proteasome inhibitors. Biochem Biophys Res Commun 1996; 224: 74–9
Adams J, Palombella VJ, Sausville EA, et al. Proteasome inhibitors: a novel class of potent and effective antitumor agents. Cancer Res 1999; 59: 2615–22
Hideshima T, Richardson P, Chauhan D, et al. The proteasome inhibitor PS-341 inhibits growth, induces apoptosis, and overcomes drug resistance in human multiple myeloma cells. Cancer Res 2001; 61: 3071–6
Hideshima T, Mitsiades C, Akiyama M, et al. Molecular mechanisms mediating antimyeloma activity of proteasome inhibitor PS-341. Blood 2003; 101: 1530–4
Pekol T, Daniels JS, Labutti J, et al. Human metabolism of the proteasome inhibitor bortezomib: identification of circulating metabolites. Drug Metab Dispos 2005; 33: 771–7
Mitsiades N, Mitsiades CS, Poulaki V, et al. Biologic sequelae of nuclear factor-kappaB blockade in multiple myeloma: therapeutic applications. Blood 2002; 99: 4079–86
Hideshima T, Chauhan D, Richardson P, et al. NF-kappa B as a therapeutic target in multiple myeloma. J Biol Chem 2002; 277: 16639–47
Pomerantz JL, Baltimore D. Two pathways to NF-kappaB. Mol Cell 2002; 10: 693–5
Kurland JF, Meyn RE. Protease inhibitors restore radiation-induced apoptosis to Bcl-2-expressing lymphoma cells. Int J Cancer 2001; 96: 327–33
Masdehors P, Merle-Beral H, Maloum K, et al. Deregulation of the ubiquitin system and p53 proteolysis modify the apoptotic response in B-CLL lymphocytes. Blood 2000; 96: 269–74
Masdehors P, Merle-Beral H, Magdelenat H, et al. Ubiquitin-proteasome system and increased sensitivity of B-CLL lymphocytes to apoptotic death activation. Leuk Lymphoma 2000; 38: 499–504
Salvat C, Aquaviva C, Jariel-Encontre I, et al. Are there multiple proteolytic pathways contributing to c-Fos, c-Jun and p53 protein degradation in vivo? Mol Biol Rep 1999; 26: 45–51
Li B, Dou QP. Bax degradation by the ubiquitin/proteasome-dependent pathway: involvement in tumor survival and progression. Proc Natl Acad Sci U S A 2000; 97: 3850–5
Kudo Y, Takata T, Ogawa I, et al. p27Kip1 accumulation by inhibition of proteasome function induces apoptosis in oral squamous cell carcinoma cells. Clin Cancer Res 2000; 6: 916–23
Drexler HC. Activation of the cell death program by inhibition of proteasome function. Proc Natl Acad Sci U S A 1997; 94: 855–60
Kitagawa H, Tani E, Ikemoto H, et al. Proteasome inhibitors induce mitochondria-independent apoptosis in human glioma cells. FEBS Lett 1999; 443: 181–6
Nawrocki ST, Carew JS, Dunner Jr K, et al. Bortezomib inhibits PKR-like endoplasmic reticulum (ER) kinase and induces apoptosis via ER stress in human pancreatic cancer cells. Cancer Res 2005; 65: 11510–9
Obeng EA, Carlson LM, Gutman DM, et al. Proteasome inhibitors induce a terminal unfolded protein response in multiple myeloma cells. Blood 2006 Jun 15; 107 (12): 4907–16
Nawrocki ST, Carew JS, Pino MS, et al. Bortezomib sensitizes pancreatic cancer cells to endoplasmic reticulum stress-mediated apoptosis. Cancer Res 2005; 65: 11658–66
Fribley A, Zeng Q, Wang CY. Proteasome inhibitor PS-341 induces apoptosis through induction of endoplasmic reticulum stress-reactive oxygen species in head and neck squamous cell carcinoma cells. Mol Cell Biol 2004; 24: 9695–704
LeBlanc R, Catley LP, Hideshima T, et al. Proteasome inhibitor PS-341 inhibits human myeloma cell growth in vivo and prolongs survival in a murine model. Cancer Res 2002; 62: 4996–5000
Cusack Jr JC, Liu R, Houston M, et al. Enhanced chemosensitivity to CPT-11 with proteasome inhibitor PS-341: implications for systemic nuclear factor-kappaB inhibition. Cancer Res 2001; 61: 3535–40
Bold RJ, Virudachalam S, McConkey DJ. Chemosensitization of pancreatic cancer by inhibition of the 26S proteasome. J Surg Res 2001; 100: 11–7
Shah SA, Potter MW, McDade TP, et al. 26S proteasome inhibition induces apoptosis and limits growth of human pancreatic cancer. J Cell Biochem 2001; 82: 110–22
Orlowski RZ, Stinchcombe TE, Mitchell BS, et al. Phase I trial of the proteasome inhibitor PS-341 in patients with refractory hematologic malignancies. J Clin Oncol 2002; 20: 4420–7
Richardson PG, Barlogie B, Berenson J, et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med 2003; 348: 2609–17
Richardson PG, Sonneveld P, Schuster MW, et al. and the Assessment of Proteasome Inhibition for Extending Remissions (APEX) Investigators. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med 2005; 352: 2487–98
Blade J, Samson D, Reece D, et al. Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation. Myeloma Subcommittee of the EBMT. European Group for Blood and Marrow Transplant. Br J Haematol 1998; 102: 1115–23
Jagannath S, Barlogie B, Berenson JR, et al. Bortezomib in recurrent and/or refractory multiple myeloma: initial clinical experience in patients with impaired renal function. Cancer 2005; 103: 1195–200
Goel A, Dispenzieri A, Greipp PR, et al. PS-341-mediated selective targeting of multiple myeloma cells by synergistic increase in ionizing radiation-induced apoptosis. Exp Hematol 2005; 33: 784–95
Oakervee HE, Popat R, Curry N, et al. PAD combination therapy (PS-341/bortezomib, doxorubicin and dexamethasone) for previously untreated patients with multiple myeloma. Br J Haematol 2005; 129: 755–62
Kawazoe Y, Nakai A, Tanabe M, et al. Proteasome inhibition leads to the activation of all members of the heat-shock-factor family. Eur J Biochem 1998; 255: 356–62
Mitsiades N, Mitsiades CS, Poulaki V, et al. Molecular sequelae of proteasome inhibition in human multiple myeloma cells. Proc Natl Acad Sci U S A 2002; 99: 14374–9
Parcellier A, Gurbuxani S, Schmitt E, et al. Heat shock proteins, cellular chaperones that modulate mitochondrial cell death pathways. Biochem Biophys Res Commun 2003; 304: 505–12
Beere HM. “The stress of dying ”: the role of heat shock proteins in the regulation of apoptosis. J Cell Sci 2004; 117: 2641–51
Igney FH, Krammer PH. Death and anti-death: tumour resistance to apoptosis. Nat Rev Cancer 2002; 2: 277–88
Martinou JC, Green DR. Breaking the mitochondrial barrier. Nat Rev Mol Cell Biol 2001; 2: 63–7
Zamzami N, Kroemer G. The mitochondrion in apoptosis: how Pandora’s box opens. Nat Rev Mol Cell Biol 2001; 2: 67–71
Nencioni A, Garuti A, Schwarzenberg K, et al. Proteasome inhibitor-induced apoptosis in human monocyte-derived dendritic cells. Eur J Immunol 2006 Mar; 36 (3): 681–9
Steel R, Doherty JP, Buzzard K, et al. Hsp72 inhibits apoptosis upstream of the mitochondria and not through interactions with Apaf-1. J Biol Chem 2004; 279: 51490–9
Mosser DD, Caron AW, Bourget L, et al. The chaperone function of hsp70 is required for protection against stress-induced apoptosis. Mol Cell Biol 2000; 20: 7146–59
Gotoh T, Terada K, Oyadomari S, et al. Hsp70-DnaJ chaperone pair prevents nitric oxide- and CHOP-induced apoptosis by inhibiting translocation of Bax to mitochondria. Cell Death Differ 2004; 11: 390–402
Ravagnan L, Gurbuxani S, Susin SA, et al. Heat-shock protein 70 antagonizes apoptosis-inducing factor. Nat Cell Biol 2001; 3: 839–43
Gurbuxani S, Schmitt E, Cande C, et al. Heat shock protein 70 binding inhibits the nuclear import of apoptosis-inducing factor. Oncogene 2003; 22: 6669–78
Gabai VL, Mabuchi K, Mosser DD, et al. Hsp72 and stress kinase c-jun N-terminal kinase regulate the bid-dependent pathway in tumor necrosis factor-induced apoptosis. Mol Cell Biol 2002; 22: 3415–24
Paul C, Manero F, Gonin S, et al. Hsp27 as a negative regulator of cytochrome c release. Mol Cell Biol 2002; 22: 816–34
Chen G, Cao P, Goeddel DV. TNF-induced recruitment and activation of the IKK complex require Cdc37 and Hsp90. Mol Cell 2002; 9: 401–10
Chen G, Goeddel DV. TNF-R1 signaling: a beautiful pathway. Science 2002; 296: 1634–5
Lewis J, Devin A, Miller A, et al. Disruption of Hsp90 function results in degradation of the death domain kinase, receptor-interacting protein (RIP), and blockage of tumor necrosis factor-induced nuclear factor-kappa B activation. J Biol Chem 2000; 275: 10519–26
Parcellier A, Schmitt E, Gurbuxani S, et al. HSP27 is a ubiquitin-binding protein involved in I-{kappa}B{alpha} proteasomal degradation. Mol Cell Biol 2003; 23: 5790–802
Yang X, Khosravi-Far R, Chang HY, et al. Daxx, a novel Fas-binding protein that activates JNK and apoptosis. Cell 1997; 89: 1067–76
Park HS, Cho SG, Kim CK, et al. Heat shock protein Hsp72 is a negative regulator of apoptosis signal-regulating kinase 1. Mol Cell Biol 2002; 22: 7721–30
Chang HY, Nishitoh H, Yang X, et al. Activation of apoptosis signal-regulating kinase 1(ASK1) by the adapter protein daxx. Science 1998; 281: 1860–3
Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell 2000; 103: 239–52
Park HS, Lee JS, Huh SH, et al. Hsp72 functions as a natural inhibitory protein of c-Jun N-terminal kinase. EMBO J 2001; 20: 446–56
Yang JY, Michod D, Walicki J, et al. Surviving the kiss of death. Biochem Pharmacol 2004; 68: 1027–31
Dougherty MK, Morrison DK. Unlocking the code of 14-3-3. J Cell Sci 2004; 117: 1875–84
Zha J, Harada H, Yang E, et al. Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14-3-3 not BCL-X(L). Cell 1996; 87: 619–28
Peso LD, Gonzalez-Garcia M, Page C, et al. Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science 1997; 278: 687–9
Kane LP, Shapiro VS, Stokoe D, et al. Induction of NF-kappaB by the Akt/PKB kinase. Curr Biol 1999; 9: 601–4
Chu ZL, McKinsey TA, Liu L, et al. Suppression of tumor necrosis factor-induced cell death by inhibitor of apoptosis c-IAP2 is under NF-kappa B control. Proc Natl Acad Sci U S A 1997; 94: 10057–62
You M, Ku PT, Hrdlickova R, et al. ch-IAP1, a member of the inhibitor-of-apoptosis protein family, is a mediator of the antiapoptotic activity of the v-Rel oncoprotein. Mol Cell Biol 1997; 17: 7328–41
Zong WX, Edelstein LC, Chen C, et al. The prosurvival Bcl-2 homolog Bfl-1/A1 is a direct transcriptional target of NF-kappa B that blocks TNFalpha -induced apoptosis. Genes Dev 1999; 13: 382–7
Heinrich PC, Behrmann I, Haan S, et al. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J 2003; 374: 1–20
Chauhan D, Pandey P, Hideshima T, et al. SHP2 mediates the protective effect of interleukin-6 against dexamethasone-induced apoptosis in multiple myeloma cells. J Biol Chem 2000; 275: 27845–50
Tassone P, Galea E, Forciniti S, et al. The IL-6 receptor super-antagonist Sant7 enhances antiproliferative and apoptotic effects induced by dexamethasone and zoledronic acid on multi- ple myeloma cells. Int J Oncol 2002; 21: 867–73
Thomas PJ, Qu BH, Pedersen PL. Defective protein folding as a basis of human disease. Trends Biochem Sci 1995; 20: 456–9
Hideshima T, Bradner JE, Wong J, et al. Small-molecule inhibition of proteasome and aggresome function induces synergistic antitumor activity in multiple myeloma. Proc Natl Acad Sci USA 2005; 102: 8567–72
Bardag-Gorce F, Riley NE, Nan L, et al. The proteasome inhibitor, PS-341, causes cytokeratin aggresome formation. Exp Mol Pathol 2004; 76: 9–16
French BA, van Leeuwen F, Riley NE, et al. Aggresome formation in liver cells in response to different toxic mechanisms: role of the ubiquitin-proteasome pathway and the frameshift mutant of ubiquitin. Exp Mol Pathol 2001; 71: 241–6
Nawrocki ST, Carew JS, Pino MS, et al. Aggresome disruption: a novel strategy to enhance bortezomib-induced apoptosis in pancreatic cancer cells. Cancer Res 2006; 66: 3773–81
Orlowski RZ. The ubiquitin proteasome pathway from bench to bedside. Hematology Am Soc Hematol Educ Program 2005, 220–5
Chauhan D, Li G, Shringarpure R, et al. Blockade of hsp27 overcomes bortezomib/proteasome inhibitor PS-341 resistance in lymphoma cells. Cancer Res 2003; 63: 6174–7
Hideshima T, Podar K, Chauhan D, et al. p38 MAPK inhibition enhances PS-341 (bortezomib)-induced cytotoxicity against multiple myeloma cells. Oncogene 2004; 23: 8766–76
Mimnaugh EG, Xu W, Vos M, et al. Simultaneous inhibition of hsp 90 and the proteasome promotes protein ubiquitination, causes endoplasmic reticulum-derived cytosolic vacuolization, and enhances antitumor activity. Mol Cancer Ther 2004; 3: 551–66
Zaarur N, Gabai VL, Porco Jr JA, et al. Targeting heat shock response to sensitize cancer cells to proteasome and Hsp90 inhibitors. Cancer Res 2006; 66: 1783–91
Robertson JD, Datta K, Biswal SS, et al. Heat-shock protein 70 antisense oligomers enhance proteasome inhibitor-induced apoptosis. Biochem J 1999; 344 Pt 2: 477–85
Chanan-Khan A, Alsina M, Carroll M, et al. Dose escalating trial of 17-AAG with bortezomib (BZ) in patients with relapsed refractory multiple myeloma (MM) [abstract]. Proc Amr Soc Clin Oncol 2005; 24: 605s
Navas TA, Nguyen AN, Hideshima T, et al. Inhibition of p38alpha MAPK enhances proteasome inhibitor-induced apoptosis of myeloma cells by modulating Hsp27, Bcl-X(L), Mcl-1 and p53 levels in vitro and inhibits tumor growth in vivo. Leukemia 2006; 20: 1017–27
Denlinger CE, Keller MD, Mayo MW, et al. Combined proteasome and histone deacetylase inhibition in non-small cell lung cancer. J Thorac Cardiovasc Surg 2004; 127: 1078–86
Catley L, Tai YT, Chauhan D, et al. Perspectives for combination therapy to overcome drug-resistant multiple myeloma. Drug Resist Updat 2005; 8: 205–18
Pei XY, Dai Y, Grant S. Synergistic induction of oxidative injury and apoptosis in human multiple myeloma cells by the proteasome inhibitor bortezomib and histone deacetylase inhibitors. Clin Cancer Res 2004; 10: 3839–52
Dai Y, Rahmani M, Dent P, et al. Blockade of histone deacetylase inhibitor-induced RelA/p65 acetylation and NF-kappaB activation potentiates apoptosis in leukemia cells through a process mediated by oxidative damage, XIAP downregulation, and c-Jun N-terminal kinase 1 activation. Mol Cell Biol 2005; 25: 5429–44
Mitsiades N, Mitsiades CS, Richardson PG, et al. Molecular sequelae of histone deacetylase inhibition in human malignant B cells. Blood 2003; 101: 4055–62
Macherla VR, Mitchell SS, Manam RR, et al. Structure-activity relationship studies of salinosporamide A (NPI-0052), a novel marine derived proteasome inhibitor. J Med Chem 2005; 48: 3684–7
Chauhan D, Catley L, Li G, et al. A novel orally active proteasome inhibitor induces apoptosis in multiple myeloma cells with mechanisms distinct from bortezomib. Cancer Cell 2005; 8: 407–19
Acknowledgements
This study was supported in part by the Myeloma Foundation of America and the Pastore Foundation. Mohamad A. Hussein has received a research grant from Millennium Pharmaceuticals, Inc. The other authors have no conflicts of interest that are directly relevant to the content of this review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cheriyath, V., Jacobs, B.S. & Hussein, M.A. Proteasome Inhibitors in the Clinical Setting. Drugs R D 8, 1–12 (2007). https://doi.org/10.2165/00126839-200708010-00001
Published:
Issue Date:
DOI: https://doi.org/10.2165/00126839-200708010-00001