Abstract
Schizophrenia is a chronic disease that typically manifests during adolescence and early adulthood. Treatment of this disease consumes a significant proportion of the healthcare budget (billions of dollars in the US). Primary management options for schizophrenia include both pharmacologic and psychosocial interventions, with antipsychotic therapy (typicals or atypicals) being the mainstay of any treatment plan. Atypical agents are recommended in current guidelines as first-line treatments for patients with newly diagnosed schizophrenia. The newer atypical antipsychotic agents have a lower propensity to cause extrapyramidal symptoms and tardive dyskinesia, and are at least as effective as typical agents.
Long-acting injectable formulations of antipsychotic drugs help to ensure drug delivery and are recommended in patients who are partially or fully noncompliant, or who prefer this formulation. The goal of using these agents is to reduce noncompliance, thus reducing the likelihood of relapse and/or hospitalization, and ultimately reducing treatment costs. Benefits associated with long-acting formulations include the maintenance of stable plasma concentrations, the reduction of overdose risks, and the establishment of regular contact between the patient and their healthcare provider. Currently, risperidone is the only atypical agent available as a long-acting injectable formulation.
In patients with schizophrenia, long-acting injectable risperidone (25–50mg every 2 weeks) is no less effective than once-daily oral risperidone, has superior efficacy to placebo over the short term, and significantly improves symptoms over the long term in patients who are symptomatically stable at baseline. Long-acting injectable risperidone is well tolerated and has a tolerability profile similar to that of oral risperidone, apart from injection-site reactions, which are generally mild and transient. This formulation of risperidone reduces the incidence of hospitalization and significantly improves health-related quality of life (HR-QOL; in particular mental health-related domains) in patients with symptomatically stable schizophrenia. Pharmacoeconomic models have indicated that long-acting injectable risperidone — relative to oral risperidone, oral olanzapine, or long-acting haloperidol — is associated with cost savings and is the dominant strategy in terms of cost effectiveness.
The efficacy and tolerability profile of long-acting injectable risperidone, its ability to reduce hospitalization rates and improve HR-QOL, and its demonstrated cost effectiveness in pharmacoeconomic models support the use of this agent in the management of schizophrenia.






Similar content being viewed by others
Notes
The use of trade names is for product identification purposes only and does not imply endorsement.
References
Falkai P, Wobrock T, Lieberman J, et al. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia, Part 1: acute treatment of schizophrenia. World J Biol Psychiatry 2005; 6(3): 132–91
National Institute for Clinical Excellence. Guidance on the use of newer (atypical) antipsychotic drugs for the treatment of schizophrenia. Technology Appraisal Guidance 2002 Jun; 43: 1–20
Mortimer AM. Novel antipsychotics in schizophrenia. Expert Opin Investig Drugs 2004 Apr; 13(4): 315–29
American Psychiatric Association. Practice guideline for the treatment ofpatients with schizophrenia, second edition [online]. Available from http://www.psych.org/psych_pract/treatg/pg/Practice%20Guidelines8904/Schizophrenia_2e.pdf [Accessed 2006 Mar 22]
Thieda P, Beard S, Richter A, et al. An economic review of compliance with medication therapy in the treatment of schizophrenia. Psychiatr Serv 2003 Apr; 54(4): 508–16
Keith SJ, Pani L, Nick B, et al. Practical application of pharmacotherapy with longacting risperidone for patients with schizophrenia. Psychiatr Serv 2004 Sep; 55(9): 997–1005
Valenstein M, Copeland LA, Owen R, et al. Adherence assessments and the use of depot antipsychotics in patients with schizophrenia. J Clin Psychiatry 2001 Jul; 62(7): 545–51
Adams CE, Fenton MK, Quraishi S, et al. Systematic meta-review of depot antipsychotic drugs for people with schizophrenia. Br J Psychiatry 2001 Oct; 179: 290–9
Nuechterlein KH, Dawson ME. A heuristic vulnerability/stress model of schizophrenic episodes. Schizophr Bull 1984; 10(2): 300–12
American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Arlington (VA): American Psychiatric Association, 2000
Beiser M, Erickson D, Fleming JA, et al. Establishing the onset of psychotic illness. Am J Psychiatry 1993 Sep; 150(9): 1349–54
Häfner H, Maurer K, Löffler W, et al. Modeling the early course of schizophrenia. Schizophr Bull 2003; 29(2): 325–40
Robinson D, Woerner MG, Alvir JM, et al. Predictors of relapse following response from a first episode of schizophrenia or schizoaffective disorder. Arch Gen Psychiatry 1999 Mar; 56(3): 241–7
McGrath J, Saha S, Welham J, et al. A systematic review of the incidence of schizophrenia: the distribution of rates and the influence of sex, urbanicity, migrant status and methodology. BMC Med 2004 Apr 28; 2: 13
Saha S, Chant D, Welham J, et al. A systematic review of the prevalence of schizophrenia. PLoS Med 2005 May; 2(5): e141
Torrey EF. Prevalence studies in schizophrenia. Br J Psychiatry 1987 May; 150: 598–608
World Health Organization. World Health Report 2001 — mental health: new understanding, new hope [online]. Available from URL: http://www.who.int/whr/2001/en/whr01_en.pdf [Accessed 2005 Apr 5]
Kestenbaum CJ. Children at risk for schizophrenia. Am J Psychother 1980 Apr; 34(2): 164–77
Lambert M, Naber D. Current issues in schizophrenia: overview of patient acceptability, functioning capacity and quality of life. CNS Drugs 2004; 18 Suppl. 2: 5–17
Awad AG, Voruganti LN. Impact of atypical antipsychotics on quality of life in patients with schizophrenia. CNS Drugs 2004; 18(13): 877–93
Rössler W, Salize HJ, van Os J, et al. Size of burden of schizophrenia and psychotic disorders. Eur Neuropsychopharmacol 2005 Aug; 15(4): 399–409
Knapp M, Mangalore R, Simon J. The global costs of schizophrenia. Schizophr Bull 2004; 30(2): 279–93
Knapp M, Chisholm D, Leese M, et al. Comparing patterns and costs of schizophrenia care in five European countries: the EPSILON study. Acta Psychiatr Scand 2002 Jan; 105: 42–54
Wu EQ, Birnbaum HG, Shi L, et al. The economic burden of schizophrenia in the United States in 2002. J Clin Psychiatry 2005 Sep; 66(9): 1122–9
Glazer WM, Johnstone BM. Pharmacoeconomic evaluation of antipsychotic therapy for schizophrenia. J Clin Psychiatry 1997; 58 Suppl. 10: 50–4
Wyatt RJ, Henter I, Leary MC, et al. An economic evaluation of schizophrenia — 1991. Soc Psychiatry Psychiatr Epidemiol 1995 Aug; 30(5): 196–205
Surles RC. Atypical antipsychotics: considerations for Medicaid coverage. Am J Manag Care 2005 Sep; 11(8 Suppl.): S248–53
Hamann J, Leucht S, Kissling W. Are the second-generation antipsychotics costeffective? A critical review on the background of different health systems. Pharmacopsychiatry 2003 Jan; 36(1): 18–26
Lasser RA, Bossie CA, Gharabawi GM, et al. Patients with schizophrenia previously stabilized on conventional depot antipsychotics experience significant clinical improvements following treatment with long-acting risperidone. Eur Psychiatry 2004 Jun; 19(4): 219–25
Farmer D, Claerhout B, Mehnert A, et al. e-STAR: the electronic schizophrenia treatment adherence registry. An electronic registry to evaluate outcomes data in patients. Value Health 2004; 7 (6 Suppl. 1): 778
Mauskopf J, Muroff M, Gibson PJ, et al. Estimating the costs and benefits of new drug therapies: atypical antipsychotic drugs for schizophrenia. Schizophr Bull 2002; 28(4): 619–35
Haycox A. Pharmacoeconomics of long-acting risperidone: results and validity of cost-effectiveness models. Pharmacoeconomics 2005; 23 Suppl. 1: 3–16
National Institute for Clinical Excellence. Schizophrenia: core interventions in the treatment and management of schizophrenia in primary and secondary care [online]. Available from URL: http://www.nice.org.uk/page.aspx?o=42424 [Accessed 2006 Mar 22]
McEvoy JP, Scheifler PL, Frances A, et al. Treatment of schizophrenia 1999: the expert consensus guideline series. J Clin Psychiatry 1999; 60 Suppl. 11: 3–80
Lehman AF, Kreyenbuhl J, Buchanan RW, et al. The Schizophrenia Patient Outcomes Research Team (PORT): updated treatment recommendations 2003. Schizophr Bull 2004; 30(2): 193–217
Bhanji NH, Chouinard G, Margolese HC. A review of compliance, depot intramuscular antipsychotics and the new long-acting injectable atypical antipsychotic risperidone in schizophrenia. Eur Neuropsychopharmacol 2004 Mar; 14(2): 87–92
Donohoe G, Owens N, O’Donnell C, et al. Predictors of compliance with neuroleptic medication among inpatients with schizophrenia: a discriminant function analysis. Eur Psychiatry 2001 Aug; 16(5): 293–8
Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 2005 Sep 22; 353(12): 1209–23
Weiden PJ, Kozma C, Grogg A, et al. Partial compliance and risk of rehospitalization among California Medicaid patients with schizophrenia. Psychiatr Serv 2004 Aug; 55(8): 886–91
O’Donnell C, Donohoe G, Sharkey L, et al. Compliance therapy: a randomised controlled trial in schizophrenia. BMJ 2003 Oct 11; 327(7419): 834
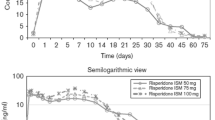
Ereshefsky L, Mannaert E. Pharmacokinetic profile and clinical efficacy of longacting risperidone: potential benefits of combining an atypical antipsychotic and a new delivery system. Drugs RD 2005; 6(3): 129–37
Kane JM. Strategies for improving compliance in treatment of schizophrenia by using a long-acting formulation of an antipsychotic: clinical studies. J Clin Psychiatry 2003; 64 Suppl. 16: 34–40
Altamura AC, Sassella F, Santini A, et al. Intramuscular preparations of antipsychotics: uses and relevance in clinical practice. Drugs 2003; 63(5): 493–512
Schooler NR. Relapse and rehospitalization: comparing oral and depot. J Clin Psychiatry 2003; 64 Suppl. 16: 14–7
Barnes TR, Curson DA. Long-term depot antipsychotics: a risk-benefit assessment. Drug Saf 1994 Jun; 10(6): 464–79
Walburn J, Gray R, Gournay K, et al. Systematic review of patient and nurse attitudes to depot antipsychotic medication. Br J Psychiatry 2001 Oct; 179: 300–7
Ereshefsky L, Mascarenas CA. Comparison of the effects of different routes of antipsychotic administration on pharmacokinetics and pharmacodynamics. J Clin Psychiatry 2003; 64 Suppl. 16: 18–23
Remington G, Chong SA. Conventional versus novel antipsychotics: changing concepts and clinical implications. J Psychiatry Neurosci 1999 Nov; 24(5): 431–41
Gardner DM, Baldessarini RJ, Waraich P. Modern antipsychotic drugs: a critical overview. CMAJ 2005 Jun 21; 172(13): 1703–11
Chakos M, Lieberman J, Hoffman E, et al. Effectiveness of second-generation antipsychotics in patients with treatment-resistant schizophrenia: a review and meta-analysis of randomized trials. Am J Psychiatry 2001 Apr; 158(4): 518–26
Leucht S, Wahlbeck K, Hamann J, et al. New generation antipsychotics versus lowpotency conventional antipsychotics: a systematic review and meta-analysis. Lancet 2003 May 10; 361(9369): 1581–9
Miller AL, Chiles JA, Chiles JK, et al. The Texas Medication Algorithm Project (TMAP) schizophrenia algorithms. J Clin Psychiatry 1999 Oct; 60(10): 649–57
Allison DB, Mentore JL, Heo M, et al. Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry 1999 Nov; 156(11): 1686–96
Newcomer JW. Abnormalities of glucose metabolism associated with atypical antipsychotic drugs. J Clin Psychiatry 2004; 65 Suppl. 18: 36–46
Casey DE. Dyslipidemia and atypical antipsychotic drugs. J Clin Psychiatry 2004; 65 Suppl. 18: 27–35
Haddad PM, Wieck A. Antipsychotic-induced hyperprolactinaemia: mechanisms, clinical features and management. Drugs 2004; 64(20): 2291–314
Janssen Pharmaceutical Ltd. Risperdal® Consta® — risperidone long-acting injection: US prescribing information. Titusville (NJ): Janssen Pharmaceutical Ltd, 2005
Janssen Pharmaceutical Products. Risperdal® (risperidone tablets/oral solution): US prescribing information. Titusville (NJ): Janssen Pharmaceutical Products, 2005
Otsuka America Pharmaceutical, Inc. Abilify® (aripiprazole) tablets: US prescribing information. Rockville (MD): Otsuka America Pharmaceutical, Inc., 2005 Dec
Eli Lilly and Company. Zyprexa® (olanzapine): US prescribing information. Indianapolis (IN): Eli Lilly and Company, 2005
Eli Lilly Nederland B.V. Zyprexa®: summary of product characteristics [online]. Available from URL: http://www.emea.eu.int/humandocs/Humans/EPAR/zyprexa/zyprexa.htm [Accessed 2006 Mar 22]
AstraZeneca Pharmaceuticals LP. Seroquel® (quetiapine fumarate) tablets: US prescribing information. Wilmington (DE): AstraZeneca Pharmaceuticals LP, 2005
Pfizer, Inc. Geodon® (ziprazone): US prescribing information. New York (NY): Pfizer, Inc., 2005 May
McKeage K, Plosker GL. Amisulpride: a review of its use in the management of schizophrenia. CNS Drugs 2004; 18(13): 933–56
Novartis Pharmaceuticals Corporation. Clozaril® (Clozapine) tablets: US prescribing information. East Hanover (NJ): Novartis Pharmaceuticals Corporation, 2005 May
Smith S, Wheeler MJ, Murray R, et al. The effects of antipsychotic-induced hyperprolactinaemia on the hypothalamic-pituitary-gonadal axis. J Clin Psychopharmacol 2002 Apr; 22(2): 109–14
Huxley NA, Rendall M, Sederer L. Psychosocial treatments in schizophrenia: a review of the past 20 years. J Nerv Ment Dis 2000 Apr; 188(4): 187–201
Royal Australian and New Zealand College of Psychiatrists. Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for the treatment of schizophrenia and related disorders. Aust N Z J Psychiatry 2005 Jan-2005 28; 39(1–2): 1–30
Lehman AF, Goldberg R, Dixon LB, et al. Improving employment outcomes for persons with severe mental illnesses. Arch Gen Psychiatry 2002 Feb; 59(2): 165–72
Swainston Harrison T, Goa KL. Long-acting risperidone: a review of its use in schizophrenia. CNS Drugs 2004; 18(2): 113–32
Chue P. Risperidone long-acting injection. Expert Rev Neurotherapeutics 2003; 3(4): 435–46
Kane JM, Eerdekens M, Lindenmayer JP, et al. Long-acting injectable risperidone: efficacy and safety of the first long-acting atypical antipsychotic. Am J Psychiatry 2003 Jun; 160: 1125–32
Chue P, Eerdekens M, Augustyns I, et al. Comparative efficacy and safety of longacting risperidone and risperidone oral tablets. Eur Neuropsychopharmacol 2005 Jan; 15(1): 111–7
Taylor DM, Young CL, Mace S, et al. Early clinical experience with risperidone long-acting injection: a prospective, 6-month follow-up of 100 patients. J Clin Psychiatry 2004 Aug; 65(8): 1076–83
Turner M, Eerdekens E, Jacko M, et al. Long-acting injectable risperidone: safety and efficacy in stable patients switched from conventional depot antipsychotics. Int Clin Psychopharmacol 2004 Jul; 19(4): 241–9
Fleischhacker WW, Eerdekens M, Karcher K, et al. Treatment of schizophrenia with long-acting injectable risperidone: a 12-month open-label trial of the first long-acting second-generation antipsychotic. J Clin Psychiatry 2003 Oct; 64(10): 1250–7
Möller HJ, Llorca PM, Sacchetti E, et al. Efficacy and safety of direct transition to risperidone long-acting injectable in patients treated with various antipsychotic therapies. Int Clin Psychopharmacol 2005 May; 20(3): 121–30
Marder SR, Conley R, Ereshefsky L, et al. Clinical guidelines: dosing and switching strategies for long-acting risperidone. J Clin Psychiatry 2003; 64 Suppl. 16: 41–6
Lauriello J, McEvoy JP, Rodriguez S, et al. Long-acting risperidone vs. placebo in the treatment of hospital inpatients with schizophrenia. Schizophr Res 2005 Jan 1; 72(2–3): 249–58
Parellada E, Andrezina R, Milanova V, et al. Patients in the early phases of schizophrenia and schizoaffective disorders effectively treated with risperidone long-acting injectable. J Psychopharmacol (Oxf) 2005 Sep; 19(5): 5–14
Gastpar M, Masiak M, Latif MA, et al. Sustained improvement of clinical outcome with risperidone long-acting injectable in psychotic patients previously treated with olanzapine. J Psychopharmacol 2005 Sep; 19(5 Suppl.): 32–8
Kissling S, Heres S, Lloyd K, et al. Direct transition to long-acting risperidone — analysis of long-term efficacy. J Psychopharmacol (Oxf) 2005 Sep; 19(5): 15–21
Chue P, Llorca PM, Duchesne I, et al. Hospitalization rates in patients during longterm treatment with long-acting risperidone injection. J Appl Res 2005; 5(2): 266–74
Leal A, Rosillon D, Mehnert A, et al. Healthcare resource utilization during 1-year treatment with long-acting, injectable risperidone. Pharmacoepidemiol Drug Saf 2004 Nov; 13(11): 811–6
Lindenmayer JP, Jarboe K, Bossie CA, et al. Minimal injection site pain and high patient satisfaction during treatment with long-acting risperidone. Int Clin Psychopharmacol 2005 Jul; 20(4): 213–21
Bloch Y, Mendlovic S, Strupinsky S, et al. Injections of depot antipsychotic medications in patients suffering from schizophrenia: do they hurt? J Clin Psychiatry 2001 Nov; 62(11): 855–9
Hay J. Complications at site of injection of depot neuroleptics. BMJ 1995 Aug 12; 311(7002): 421
McHorney CA, Ware Jr JE, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care 1993 Mar; 31(3): 247–63
Nasrallah HA, Duchesne I, Mehnert A, et al. Health-related quality of life in patients with schizophrenia during treatment with long-acting, injectable risperidone. J Clin Psychiatry 2004 Apr; 65(4): 531–6
Fleischhacker WW, Rabinowitz J, Kemmler G, et al. Perceived functioning, wellbeing and psychiatric symptoms in patients with stable schizophrenia treated with long-acting risperidone for 1 year. Br J Psychiatry 2005 Aug; 187: 131–6
De Graeve D, Smet A, Mehnert A, et al. Long-acting risperidone compared with oral olanzapine and haloperidol depot in schizophrenia: a Belgian cost-effectiveness analysis. Pharmacoeconomics 2005; 23 Suppl. 1: 35–47
Llorca PM, Miadi-Fargier H, Lançon C, et al. Cost-effectiveness analysis of schizophrenic patient care settings: impact of an atypical antipsychotic under long-acting injection formulation [in French]. Encephale 2005 Mar–Apr 30; 31(2): 235–46
Chue PS, Heeg BMS, Buskens E, et al. Modelling the impact of compliance on the costs and effects of long-acting risperidone in Canada. Pharmacoeconomics 2005; 23 Suppl. 1: 62–74
Edwards NC, Rupnow MFT, Pashos CL, et al. Cost-effectiveness model of longacting risperidone in schizophrenia in the US. Pharmacoeconomics 2005; 23(3): 299–314
Laux G, Heeg BMS, Van Hout BA, et al. Costs and effects of long-acting risperidone compared with oral atypical and conventional depot formulations in Germany. Pharmacoeconomics 2005; 23 Suppl. 1: 49–61
Author information
Authors and Affiliations
Corresponding author
Additional information
Various sections of the manuscript reviewed by: A.C. Altamura, Department of Psychiatry, University of Milan, Milan, Italy; N.H. Bhanji, Peter Lougheed Hospital, Calgary, Alberta, Canada; P.S. Chue, Department of Psychiatry, University of Alberta, Edmonton, Alberta, Canada; B. Dell’Osso, Department of Psychiatry, University of Milan, Milan, Italy; J.M. Kane, The Zucker Hillside Hospital, Glen Oaks, New York, USA; R.C. Love, University of Maryland, Baltimore, Maryland, USA; A.M. Mortimer, University of Hull, Hull, England; G.L. Stimmel, USC Schools of Pharmacy and Medicine, Los Angeles, California, USA.
Data Selection
Sources: Medical literature published in any language since 1980 on ‘risperidone’, identified using MEDLINE, supplemented by AdisBase (a proprietary database of Adis International). Additional references were identified from the reference lists of published articles. Bibliographical information, including contributory unpublished data, was also requested from the company developing the drug.
Search strategy: MEDLINE search terms were ‘risperidone’ and (‘intramuscular’ or ‘depot’ or ‘injectable’ or ‘injection’ or ‘long-acting’) and (‘guidelines’ or ‘decision-making’ or ‘health-policy’ or ‘managed-care-programs’ or ‘epidemiology’ or ‘outcome-assessment-health-care’ or ‘clinical-protocols’ or ‘guideline in pt’ or ‘polic* in ti’ or ‘expert panel’ or ‘utilization review’ or ‘algorithms’ or ‘disease management’ or ‘quality of life’) or ‘schizophrenia’ and ‘review in pt’. AdisBase search terms were ‘risperidone’ and (‘intramuscular’ or ‘depot’ or ‘injectable’ or ‘injection’ or ‘long-acting’) and (‘guideline' or ‘guideline-utilization' or ‘practice-guideline’ or ‘disease-management-programs' or ‘treatment-algorithms’ or ‘reviews-on-treatment' or ‘drug-evaluations’ or ‘epidemiology’ or ‘cost-of-illness’ or ‘pathogenesis’) or ‘schizophrenia’ and (‘review’ or ‘clinical-study’). Searches were last updated on March 16, 2006.
Selection: Studies in patients with schizophrenia who received long-acting injectable risperidone. Inclusion of studies was based mainly on the methods section of the trials. When available, large, well controlled trials with appropriate statistical methodology were preferred. Relevant pharmacodynamic, pharmacokinetic, pharmacoeconomic, and epidemiologic data are also included.
Index terms: Long-acting injectable risperidone, schizophrenia, therapeutic use, tolerability, disease management, review on treatment.
Rights and permissions
About this article
Cite this article
Curran, M.P., Keating, G.M. Management of Schizophrenia. Dis-Manage-Health-Outcomes 14, 107–125 (2006). https://doi.org/10.2165/00115677-200614020-00006
Published:
Issue Date:
DOI: https://doi.org/10.2165/00115677-200614020-00006