Abstract
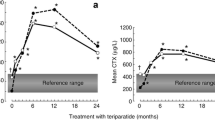
Recombinant teriparatide (Forteo®; Forsteo®) is an anabolic (bone-forming) agent. Studies have shown that subcutaneous teriparatide 20 μg/day is effective in women with postmenopausal osteoporosis, men with idiopathic or hypogonadal osteoporosis, and patients with glucocorticoid-induced osteoporosis. Teriparatide improves bone mineral density (BMD) and alters the levels of bone formation and resorption markers; histomorphometric studies have shown teriparatide-induced effects on bone structure, strength, and quality.
Subcutaneous teriparatide 20 μg/day administered over a treatment period of 11–21 months was effective in reducing the risk of fractures and improving BMD in men with idiopathic or hypogonadal osteoporosis, women with postmenopausal osteoporosis, and patients with glucocorticoid-induced osteoporosis. Furthermore, the beneficial effects of teriparatide on vertebral fracture prevention and BMD appear to persist following treatment cessation. Teriparatide is generally well tolerated and treatment compliance rates are favorable. However, current limitations on the length of treatment and the high acquisition cost mean that teriparatide is best reserved for the treatment of patients with osteoporosis at high risk of fracture, or for patients with osteoporosis who have unsatisfactory responses to or intolerance of other osteoporosis therapies.
Similar content being viewed by others
References
Blick SKA, Dhillon S, Keam SJ. Teriparatide: a review of its use in osteoporosis. Drugs 2008; 68(18): 2709–37
Eli Lilly and Company. Forteo™ [teriparatide (rDNA origin)] injection: medical review(s) [online]. Available from URL: http://www.fda.gov/cder/foi/nda/2002/21-318_FORTEO_Prntlbl.pdf [Accessed 2008 Jul 3]
Eli Lilly. Forteo™ teriparatide (rDNA) injection 750μg/3mL [online]. Available from URL: http://www.fda.gov/cder/foi/label/2008/021318s015lbl.pdf [Accessed 2008 Apr 3]
Brixen KT, Christensen PM, Ejersted C, et al. Teriparatide (biosynthetic human parathyroid hormone 1-34): a new paradigm in the treatment of osteoporosis. Basic Clin Pharmacol Toxicol 2004; 94(6): 260–70
Buxton EC, Yao W, Lane NE. Changes in serum receptor activator of nuclear factor-kappaB ligand, osteoprotegerin, and interleukin-6 levels in patients with glucocorticoid-induced osteoporosis treated with human parathyroid hormone (1-34). J Clin Endocrinol Metab 2004 Jul; 89(7): 3332–6
Girotra M, Rubin MR, Bilezikian JP. The use of parathyroid hormone in the treatment of osteoporosis. Rev Endo Metab Dis 2006; 7(1–2): 113–21
McClung MR, San Martin J, Miller PD, et al. Opposite bone remodeling effects of teriparatide and alendronate in increasing bone mass [published erratum appears in Arch Intern Med 2005 Oct 10; 165 (18): 2120]. Arch Intern Med 2005 Aug 8; 165(15): 1762–8
Arlot M, Meunier PJ, Boivin G, et al. Differential effects of teriparatide and alendronate on bone remodeling in postmenopausal women assessed by histomorphometric parameters. J Bone Miner Res 2005 Jul; 20(7): 1244–53
Dobnig H, Sipos A, Jiang Y, et al. Early changes in biochemical markers of bone formation correlate with improvements in bone structure during teriparatide therapy. J Clin Endocrinol Metab 2005 Jul; 90(7): 3970–7
US FDA Center for Drug Evaluation and Research. Clinical pharmacology and biopharmaceutics review(s) [online]. Available from URL: http://www.fda.gov/cder/foi/nda/2002/21-318_FORTEO_BioPharmr.pdf [Accessed 2008 Apr 14]
European Medicines Agency. Teriparatide (Forsteo) 20μg/80μL: summary of product characteristics [online]. Available from URL: http://www.emea.europa.eu/humandocs/PDFs/EPAR/forsteo/H-425-PI-en.pdf [Accessed 2008 Apr 29]
Neer RM, Arnaud CD, Zanchetta JR, et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 2001 May 10; 344(19): 1434–41
Miller PD, Schwartz EN, Chen P, et al. Teriparatide in postmenopausal women with osteoporosis and mild or moderate renal impairment. Osteoporos Int 2007 Jan; 18(1): 59–68
Boonen S, Marin F, Mellstrom D, et al. Safety and efficacy of teriparatide in elderly women with established osteoporosis: bone anabolic therapy from a geriatric perspective. J Am Geriatr Soc 2006 May; 54(5): 782–9
Delmas PD, Licata AA, Reginster JY, et al. Fracture risk reduction during treatment with teriparatide is independent of pretreatment bone turnover. Bone 2006 Aug; 39(2): 237–43
Gallagher JC, Genant HK, Crans GG, et al. Teriparatide reduces the fracture risk associated with increasing number and severity of osteoporotic fractures. J Clin Endocrinol Metab 2005 Mar; 90(3): 1583–7
Marcus R, Wang O, Satterwhite J, et al. The skeletal response to teriparatide is largely independent of age, initial bone mineral density, and prevalent vertebral fractures in postmenopausal women with osteoporosis. J Bone Miner Res 2003 Jan; 18(1): 18–23
Hwang JS, Tu ST, Yang TS, et al. Teriparatide vs. calcitonin in the treatment of Asian postmenopausal women with established osteoporosis. Osteoporos Int 2006; 17(3): 373–8
Kung AW, Pasion EG, Sofiyan M, et al. A comparison of teriparatide and calcitonin therapy in postmenopausal Asian women with osteoporosis: a 6-month study. Curr Med Res Opin 2006 May; 22(5): 929–37
Deal C, Omizo M, Schwartz EN, et al. Combination teriparatide and raloxifene therapy for postmenopausal osteoporosis: results from a 6-month double-blind placebo-controlled trial. J Bone Miner Res 2005 Nov; 20(11): 1905–11
Eastell R, Hadji P, Farrerons J, et al. Comparison of 3 sequential treatment regimens of teriparatide: final results from the EUROFORS study [abstract no. 1265]. J Bone Miner Res 2006 Sep; 21Suppl. 1: 70
Minne H, Audran M, Simões ME, et al. Bone density after teriparatide in patients with or without prior antiresorptive treatment: one-year results from the EUROFORS study. Curr Med Res Opin. Epub 2008 Oct 4
Cosman F, Wermers RA, Recknor C, et al. Efficacy of adding teriparatide versus switching to teriparatide in postmenopausal women with osteoporosis previously treated with raloxifene or alendronate [abstract no. O423]. J Bone Miner Res 2007; 22Suppl. 1: 89
Prince R, Sipos A, Hossain A, et al. Sustained nonvertebral fragility fracture risk reduction after discontinuation of teriparatide treatment. J Bone Miner Res 2005 Sep; 20(9): 1507–13
Adami S, Martin JS, Munoz-Torres M, et al. Effect of raloxifene after recombinant teriparatide [hPTH (1-34)] treatment in postmenopausal women with osteoporosis. Osteoporos Int 2008 Jan; 19(1): 87–94
Orwoll ES, Scheele WH, Paul S, et al. The effect of teriparatide [human parathyroid hormone (1-34)] therapy on bone density in men with osteoporosis. J Bone Miner Res 2003; 18(1): 9–17
Saag KG, Shane E, Boonen S, et al. Teriparatide or alendronate in glucocorticoid-induced osteoporosis. N Engl J Med 2007 Nov 15; 357(20): 2028–39
Stevenson M, Jones ML, De Nigris E, et al. A systematic review and economic evaluation of alendronate, etidronate, risedronate, raloxifene and teriparatide for the prevention and treatment of postmenopausal osteoporosis. Health Technol Assess 2005; 9(22): 1–160
UK National Institute for Clinical Excellence. Bisphosphonates (alendronate, etidronate, risedronate), selective oestrogen receptors modulators (raloxifene) and parathyroid hormone (teriparatide) for the secondary prevention of osteoporotic fragility fractures in postmenopausal women (technology appraisal 87) [online]. Available from URL: http://www.nice.org.uk/nicemedia/pdf/TA087guidance.pdf [Accessed 2008 Jul 3]
Pfister AK, Welch CA, Lester MD, et al. Cost-effectiveness strategies to treat osteoporosis in elderly women. South Med J 2006 Feb; 99(2): 123–31
Lundkvist J, Johnell O, Cooper C, et al. Economic evaluation of parathyroid hormone in the treatment of osteoporosis in postmenopausal women. Osteoporos Int 2006 Feb 1; 17(2): 201–11
Liu H, Michaud K, Nayak S, et al. The cost-effectiveness of therapy with teriparatide and alendronate in women with severe osteoporosis. Arch Intern Med 2006; 166(11): 1209–17
European Medicines Agency. Scientific discussion [online]. Available from URL: s p://www RLemea.europa.eu/humandocs/PDFs/EPAR/forsteo/659802en6. pdf [Accessed 2008 Apr 3]
Harper KD, Krege JH, Marcus R, et al. Comments on initial experience with teriparatide in the United States [letter]. Curr Med Res Opin 2006; 22(10): 1927
Author information
Authors and Affiliations
Corresponding author
Additional information
Adapted and reproduced from Drugs 2008; 68 (18): 2709–2737. The full text article[1] was reviewed by: A. Anastasilakis, Department of Endocrinology, Military Hospital, Thessaloniki, Greece; M. Haas, Medical University Vienna, Vienna, Austria; M.P. Kane, Department of Pharmacy Practice, Albany College of Pharmacy, Albany, New York, USA; K. Klaushofer, Ludwig Boltzmann Institute of Osteology, Hanusch Hospital, Vienna, Austria; B. Obermayer-Pietsch, Internal Medicine, Medical University Graz, Graz, Austria; and J.F. Whitfield, Institute for Biological Sciences, National Research Council of Canada, Ottawa, Ontario, Canada. The manufacturer of the agent under review was offered an opportunity to comment on the original article during the peer review process. Changes based on any comments received were made on the basis of scientific and editorial merit. The preparation of the original article and this spotlight was not supported by any external funding
Rights and permissions
About this article
Cite this article
Blick, S.K., Dhillon, S. & Keam, S.J. Spotlight on Teriparatide in Osteoporosis. BioDrugs 23, 197–199 (2009). https://doi.org/10.2165/00063030-200923030-00006
Published:
Issue Date:
DOI: https://doi.org/10.2165/00063030-200923030-00006