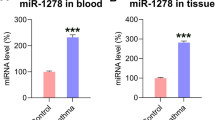
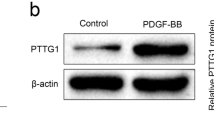
Abstract
The phosphatidylinositol 3-kinase (PI3K) signaling pathway plays a critical role in regulating cell growth, proliferation, survival, and motility. Structural alterations, e.g. airway remodeling, in asthma and chronic obstructive pulmonary disease (COPD) are associated with increased airway smooth muscle (ASM) cell growth and proliferation due to the frequent stimulation of ASM by inflammatory mediators, contractile agonists, and growth factors. The critical role of the PI3K signaling pathway in regulating ASM cell growth and proliferation is well established. However, recent discovery of the tumor suppressor proteins tuberous sclerosis complex 1 (TSC1) and TSC2, also known as hamartin and tuberin, as downstream effectors of PI3K and upstream regulators of the mammalian target of rapamycin (mTOR) and S6 kinase 1 (S6K1) shed a new light on the PI3K signaling cascade in regulating cell growth and proliferation. The activity of TSC1/TSC2 is regulated by growth factors, nutrients, and energy; thus, TSC1/TSC2 serves as a signaling module for protein translational regulation, cell cycle progression, and cell size, which are key events controlling cell growth and proliferation. This article highlights the potential contribution of the PI3K-TSC1/TSC2-mTOR/S6K1 pathway in smooth muscle remodeling. Pharmacologic manipulation of this signaling pathway could have a major impact on treatment of asthma and COPD.

Similar content being viewed by others
References
Boushey HA, Holtzman MJ, Sheller JR, et al. State of the art: bronchial hyperreactivity. Am Rev Resp Dis 1980; 121: 389–413
Woolcock AJ. Asthma: what are the important experiments? State of the art/conference summary. Am Rev Resp Dis 1989; 138: 730–44
Rennard SI. Chronic obstructive pulmonary disease: linking outcomes and pathobiology of disease modification. Proc Am Thoracic Soc 2006; 3(3): 276–80
Holgate ST. Exacerbations: the asthma paradox. Am J Resp Crit Care Med 2005; 172(8): 941–3
Barnes N. Outcome measures in asthma. Thorax 2000; 55 Suppl.: 70S–74
Panettieri RA, Grunstein MM. Airway smooth muscle hyperplasia and hypertrophy. In: Barnes PJ, Grunstein MM, Leff AR, et al. editors. Asthma. Philadelphia (PA): Lippincott-Raven; 1997: 823–42
Homer RJ, Elias JA. Airway remodeling in asthma: therapeutic implications of mechanisms. Physiology 2005; 20(1): 28–35
Jeffery PK. Remodeling in asthma and chronic obstructive lung disease. Am J Resp Crit Care Med 2001; 164 (10 Suppl.): 28S–38
Jeffery PK. Remodeling and inflammation of bronchi in asthma and chronic obstructive pulmonary disease. Proc Am Thoracic Soc 2004; 1(3): 176–83
Aoshiba K, Nagai A. Differences in airway remodeling between asthma and chronic obstructive pulmonary disease. Clin Rev Allergy Immunol 2004; 1: 35–43
Black JL. Asthma: more muscle cells or more muscular cells? Am J Respir Crit Care Med 2004; 169(9): 980–1
Dunnill MS. The pathology of asthma, with special reference to the changes in the bronchial mucosa. J Clin Pathol 1960; 13: 27–33
Dunnill MS, Massarella GR, Anderson JA. A comparison of the quantitative anatomy of the bronchi in normal subjects, in status asthmaticus, in chronic bronchitis, and in emphysema. Thorax 1969; 24: 176–9
Hossain S. Quantitative measurement of bronchial muscle in asthma. Am Rev Resp Dis 1973; 107: 99–109
Ebina M, Takahashi T, Chiba T, et al. Cellular hypertrophy and hyperplasia of airway smooth muscle underlying bronchial asthma: a 3-D morphometric study. Am Rev Resp Dis 1993; 148: 720–6
Lazaar AL, Panettieri RA. Airway smooth muscle as an immunomodulatory cell: a new target for pharmacotherapy? Curr Opin Pharmacol 2001; 1: 259–64
Woodruff PG, Dolganov GM, Ferrando RE, et al. Hyperplasia of smooth muscle in mild to moderate asthma without changes in cell size or gene expression. Am J Resp Crit Care Med 2004; 169(9): 1001–6
Woodruff PG. Airway remodeling in asthma. Semin Resp Crit Care Med 2002; 23: 361–8
Benayoun L, Druilhe A, Dombret M-C, et al. Airway structural alterations selectively associated with severe asthma. Am J Resp Crit Care Med 2003; 167(10): 1360–8
Panettieri J, Reynold A. Airway smooth muscle: an immunomodulatory cell. J Allergy Clin Immunol 2002; 110 Suppl.: S269–74
Thomas CF, Limper AH. Phosphatidylinositol kinase regulation of airway smooth muscle cell proliferation. Am J Resp Cell Mol Biol 2000; 23(4): 429–30
Page K, Hershenson MB. Mitogen-activated signaling and cell cycle regulation in airway smooth muscle. Front Biosci 2000; 5: d258–67
Condliffe AM, Cadwallader KA, Walker TR, et al. Phosphoinositide 3-kinase: a critical signaling event in pulmonary cells. Resp Res 2000; 1: 24–9
Halayko AJ, Kartha S, Stelmack GL, et al. Phophatidylinosito1–3 kinase/mammalian target of rapamycin/p70s6k regulates contractile protein accumulation in airway myocyte differentiation. Am J Resp Cell Molec Biol 2004; 31(3): 266–75
Vanhaesebroeck B, Leevers SJ, Ahmadi K, et al. Synthesis and function of 3-phosphorylated inositol lipids. Ann Rev Biochem 2001; 70(1): 535–602
Maehama T, Taylor GS, Dixon JE. PTEN and myotubularin: novel phosphoinositide phosphatases. Ann Rev Biochem 2001; 70(1): 247–79
Vanhaesebroeck B, Ali K, Bilancio A, et al. Signalling by PI3K isoforms: insights from gene-targeted mice. Trends Biochem Sci 2005; 30(4): 194–204
Chantry D, Vojtek A, Kashishian A, et al. p110δ, a novel phosphatidylinositol 3-kinase catalytic subunit that associates with p85 and is expressed predominantly in leukocytes. J Biol Chem 1997; 272: 19236–41
Stoyanov B, Volonia S, Hanck T, et al. Cloning and characterization of a G protein-activated human phosphoinositide 3-kinase. Science 1995; 269: 690–3
Domin J, Gaidarov I, Smith MEK, et al. The class II phosphoinositide 3-kinase PI3K-α is concentrated in the trans-Golgi network and present in clatrin-coated vesicles. J Biol Chem 2000; 275: 11943–50
Volinia S, Dhand R, Vanhaesebroeck B, et al. A human phosphatidylinositol 3-kinase complex related to the yeast Vps34p-Vpsl5p protein sorting system. EMBO J 1995; 14: 3339–48
Deane JA, Fruman DA. Phosphoinositide 3-kinase: diverse roles in immune cell activation. Ann Rev Immunol 2004; 22(1): 563–98
Kozma SC, Thomas G. Regulation of cell size in growth, development and human disease. BioEssays 2002; 24: 65–71
Katso R, Okkenhaug K, Ahmadi K, et al. Cellular function of phosphoinositide 3-kinases: implications for development, homeostasis, and cancer. Ann Rev Cell Develop Biol 2001; 17(1): 615–75
Bi L, Okabe I, Bernard DJ, et al. Proliferative defect and embryonic lethality in mice homozygous for a deletion in the p110α subunit of phosphoinositide 3-kinase. J Biol Chem 1999; 274: 10963–8
Fruman DA, Snapper SB, Yballe CM, et al. Impaired B cell development and proliferation in absence of phosphoinositide 3-kinase p85α. Science 1999; 283: 393–7
Shioi T, Kang PM, Douglas PS, et al. The conserved phosphoinositide 3-kinase pathway determines heart size in mice. EMBO J 2000; 19: 2537–48
Di Cristofano A, Pandolfi PP. The multiple roles of PTEN in tumor suppression. Cell 2000; 100: 387–90
Krymskaya VP. Tumor suppressors hamartin and tuberin: intracellular signaling. Cell Signal 2003; 15: 729–39
Carsillo T, Astrinidis A, Henske EP. Mutations in the tuberous sclerosis complex gene TSC2 are a cause of sporadic pulmonary lymphangioleiomyomatosis. Proc Natl Acad Sci U S A 2000; 97: 6085–90
Moses J. LAM and other diseases characterized by smooth muscle proliferation. New York: Marcel Dekker, Inc., 1999
Johnson SR, Tattersfield AE. Lymphangioleiomyomatosis. Semin Resp Crit Care Med 2002; 23: 85–92
Sullivan EJ. Lymphangioleiomyomatosis. Chest 1998; 114: 1689–703
Krymskaya VP, Shipley JM. Lymphangioleiomyomatosis: a complex tale of serum response factor-mediated tissue inhibitor of metalloproteinase-3 regulation. Am J Resp Cell Molec Biol 2003; 28(5): 546–50
Inoki K, Corradetti MN, Guan K-L. Dysregulation of the TSC-mTOR pathway in human disease. Nat Genet 2005; 37(1): 19–24
Findlay GM, Harrington LS, Lamb RF. TSC1-2 tumor suppressor and regulation of mTOR signalling: linking cell growth and proliferation? Curr Opin Genet Develop 2005; 15(1): 69–76
Astrinidis A, Henske EP. Tuberous sclerosis complex: linking growth and energy signaling pathways with human disease. Oncogene 2005; 24(50): 7475–81
Nobukini T, Thomas G. The mTOR/S6K signaling pathway: the role of the TSC1/2 tumor suppressor complex and the proto-oncogene Rheb. Novartis Foundation Symposium 2005; 262: 148–59
Lamb RF, Roy C, Diefenbach TJ, et al. The TSC1 tumor suppressor hamartin regulates cell adhesion through ERM proteins and the GTPase Rho. Nat Cell Biol 2000; 2: 281–7
Goncharova E, Goncharov D, Noonan D, et al. TSC2 modulates actin cytoskeleton and focal adhesion through TSC1-binding domain and the Rac1 GTPase. J Cell Biol 2004; 167(6): 1171–82
Onda H, Lueck A, Marks PW, et al. Tsc2+/- mice develop tumors in multiple sites that express gelsolin and are influenced by genetic background. J Clin Invest 1999; 104: 687–95
Kobayashi E, Minowa O, Kuno J, et al. Renal carcinogenesis, hepatic hemangiomatosis, and embryonic lethality caused by a germ-line Tsc2 mutation in mice. Cancer Res 1999; 59: 1206–11
Kobayashi T, Minowa O, Sugitani Y, et al. A germ-line Tsc1 mutation causes tumor development and embryonic lethality that are similar, but not identical to, those caused by Tsc2 mutation in mice. Proc Natl Acad Sci U S A 2001; 98(15): 8762–7
Kwiatkowski DJ, Zhang H, Bandura JL, et al. A mouse model of TSC 1 reveals sex-dependent lethality from liver hemangiomas, and up-regulation of p70S6 kinase activity in Tsc1 null cells. Hum Mol Genet 2002; 11: 525–34
Goncharova EA, Goncharov DA, Eszterhas A, et al. Tuberin regulates p70 S6 kinase activation and ribosomal protein S6 phosphorylation: a role for the TSC2 tumor suppressor gene in pulmonary lymphangioleiomyomatosis. J Biol Chem 2002; 277: 30958–67
McManus EJ, Alessi DR. TSC1-TSC2: a complex tale of PKB-mediated S6K regulation. Nat Cell Biol 2002; 4: E214–6
Manning BD, Tee AR, Longdon MN, et al. Identification of the tuberous sclerosis scomplex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/Akt/PKB pathway. Mol Cell 2002; 10: 151–62
Castro AF, Rebhun JF, Clark GG, et al. Rheb binds TSC2 and promotes S6 kinase activation in a rapamycin- and farnesylation-dependent manner. J Biol Chem 2003; 278: 32493–6
Garami A, Zwartruis FJT, Nobukuni T, et al. Insulin activation of Rheb, a mediator of mTOR/S6K/4E-BP signaling, is inhibited by TSC1 and 2. Mol Cell 2003; 11: 1457–66
Inoki K, Li Y, Xu T, et al. Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Devel 2003; 17(15): 1829–34
Tee AR, Manning BD, Roux PP, et al. Tuberous sclerosis complex gene products, tuberin and hamartin, control mTOR signaling by acting as a GTPase-activating protein complex toward Rheb. Curr Biol 2003; 13(15): 1259–68
Tee AR, Anjum R, Blenis J. Inactivation of the tuberous sclerosis complex-1 and -2 gene products occurs by phosphoinositide 3-kinase (PI3K)/Akt/PKB-dependent and -independent phosphorylation of tuberin. J Biol Chem 2003; 278: 37288–96
Long X, Lin Y, Ortiz-Vega S, et al. Rheb binds and regulates the mTOR kinase. Curr Biol 2005; 15(8): 702–13
Long X, Ortiz-Vega S, Lin Y, et al. Rheb binding to mammalian target of rapamycin (mTOR) is regulated by amino acid sufficiency. J Biol Chem 2005; 280(25): 23433–6
Pan D, Dong J, Zhang Y, et al. Tuberous sclerosis complex: from Drosophila to human disease. Trends Cell Biol 2004; 14(2): 78–85
Inoki K, Ouyang H, Li Y, et al. Signaling by target of rapamycin proteins in cell growth control. Microbiol Mol Biol Rev 2005; 69(1): 79–100
Schmelzte T, Hall MN. TOR, a central controller of cell growth. Cell 2000; 103: 253–62
Sarbassov DD, Ali SM, Sabatini DM. Growing roles for the mTOR pathway. Curr Opin Cell Biol 2005; 17(6): 596–603
Brunn GJ, Fadden P, Haystead TAJ, et al. The mammalian target of rapamycin phosphorylates sites having a (Ser/Thr)-pro motif and is activated by antibodies to a region near its COOH terminus. J Biol Chem 1997; 272(51): 32547–50
Loewith R, Jacinto E, Wullschleger S, et al. Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol Cell 2002; 10(3): 457–68
Sarbassov DD, Siraj MA, Kim D-H. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol 2004; 14(14): 1296–302
Jacinto E, Loewith R, Schmidt A, et al. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat Cell Biol 2004; 6(11): 1122–8
Sarbassov DD, Guertin DA, Ali SM, et al. Phosphorylation and regulation of Akt/ PKB/PKB by the Rictor-mTOR complex. Science 2005; 307(5712): 1098–101
Yang Q, Inoki K, Kim E, et al. TSC1/TSC2 and Rheb have different effects on TORC1 and TORC2 activity. Proc Natl Acad Sci U S A 2006; 103(18): 6811–6
Sigal NH, Lin CS, Siekierka JJ. Inhibition of human T-cell activation by FK 506, rapamycin, and cyclosporine A. Transplant Proc 1991; 23: 1–5
Chung J, Kuo CJ, Crabtree GR, et al. Rapamycin-FKBP specifically blocks growth-dependent activation of and signaling by the 70 kd S6 protein kinases. Cell 1992; 69: 1227–36
Kuo CJ, Chung J, Fiorentino DF, et al. Rapamycin selectively inhibits interleukin-2 activation of p70 S6 kinase. Nature 1992; 358: 70–3
Price DJ, Grove JR, Calvo V, et al. Rapamycin-induced inhibition of the 70-kilodalton S6 protein kinase. Science 1992; 257: 973–7
Burnett PE, Barrow RK, Cohen NA, et al. RAFT1 phosphorylation of the translational regulators p70 S6 kinase and 4E-BP1. Proc Natl Acad Sci U S A 1998; 95: 1432–7
Brunn GJ, Hudson CC, Sekulic A, et al. Phosphorylation of the translational repressor PHAS-I by mammalian target of rapamycin. Science 1997; 277: 99–101
Avruch J. Insulin signal transduction through protein kinase cascades. Mol Cell Biochem 1998; 182: 31–48
Gebauer F, Hentze MW. Molecular mechanisms of translational control. Nat Rev Mol Cell Biol 2004; 5: 827–35
Fingar DC, Blenis J. Target of rapamycin (TOR): an integrator of nutrient and growth factor signals and coordinator of cell growth and cell cycle progression. Oncogene 2004; 23: 3151–71
Gout I, Minami T, Hara K, et al. Molecular cloning and characterization of a novel p70 S6 kinase, p70 S6 kinase β containing proline-rich region. J Biol Chem 1998; 273: 30061–4
Nurse P. Cyclin dependent kinases and cell cycle control. Biosci Rep 2002; 22: 487–99
Nurse P. Genetic control of cell size at cell division in yeast. Nature 1975; 256: 547–51
Johnston GC, Pringle JR, Hartwell LH. Coordination of growth with cell division in the yeast Sacchromyces cerevisiae. Exp Cell Res 1977; 105: 79–98
Neufeld TP, de la Cruz AFA, Johnston LA, et al. Coordination of growth and cell division in the Drosophila wing. Cell 1998; 93(7): 1183–93
Weigmann K, Cohen S, Lehner C. Cell cycle progression, growth and patterning in imaginai discs despite inhibition of cell division after inactivation of Drosophila Cdc2 kinase. Development 1997; 124(18): 3555–63
Montagne J, Stewart MJ, Stocker H, et al. Drosophila S6 kinase: a regulator of cell size. Science 1999; 285: 2126–9
Pende M, Um SH, Mieulet V, et al. S6K1-/-/S6K2-/- mice exhibit perinatal lethality and rapamycin-sensitive 5′-terminal oligopyrimidine mRNA translation and reveal a mitogen-activated protein kinase-dependent s6 kinase pathway. Mol Cell Biol 2004; 24(8): 3112–24
Ohanna M, Sobering AK, Lapointe T, et al. Atrophy of S6K1-/- skeletal muscle cells reveals distinct mTOR effectors for cell cycle and size control. Nat Cell Biol 2005; 7(3): 286–94
Billington CK, Kong KC, Bhattacharyya R, et al. Cooperative regulation of p70s6 kinase by receptor tyrosine kinases and G protein-coupled receptors augments airway smooth muscle growth. Biochemistry 2005; 44(44): 14595–605
Billington CK, Penn RB. Signaling and regulation of G protein-coupled receptors in airway smooth muscle. Resp Res 2003; 4(2): 1–23
Vanhaesebroeck B, Waterfield MD. Signaling by distinct classes of phosphoinositide 3-kinases. Exp Cell Res 1999; 253: 239–54
Leopoldt D, Hanck T, Exner T, et al. Gβy stimulates phosphoinositide 3-kinase-γ by direct interaction with two domains of catalytic p110 subunit. J Biol Chem 1998; 273: 7024–9
Lopez-Ilasaca M, Crespo P, Pellici PG, et al. Linkage of G protein-coupled receptors to the MAPK signaling pathway through PI 3-kinase γ. Science 1997; 275: 394–7
Dekker LV, Segal AW. Signals to move cells. Science 2000; 287: 982–5
Krymskaya VP, Penn RB, Orsini MJ, et al. Phosphatidylinositol 3-kinase mediates mitogen-induced human airway smooth muscle cell proliferation. Am J Physiol 1999; 277: L65–78
Krymskaya VP, Hoffman R, Eszterhas A, et al. EGF activates ErbB2 and stimulates phosphatidylinositol 3-kinase in human airway smooth muscle cells. Am J Physiol 1999; 276: L246–55
Orsini MJ, Krymskaya VP, Eszterhas AJ, et al. MAPK superfamily activation in human airway smooth muscle cell: mitogenesis requires prolonged p42/p44 activation. Am J Physiol 1999; 277: L479–88
Ammit AJ, Kane SA, Panettieri RA. Activation of K-p21ras and N-p21ras, but not H-p21ras, is necessary for mitogen-induced human airway smooth-muscle proliferation. Am J Resp Cell Molec Biol 1999; 21: 719–27
Scott PH, Belham CM, Al-Hafidh J, et al. A regulatory role for cAMP in phosphatidylinositol 3-kinase/p70 ribosomal S6 kinase-mediated DNA synthesis in platelet-derived-growth-factor-stimulated bovine airway smooth-muscle cells. Biochem J 1996; 318: 965–71
Krymskaya VP, Orsini MJ, Eszterhas AJ, et al. Mechanisms of proliferation synergy by receptor tyrosine kinase and G protein-coupled receptor activation in human airway smooth muscle. Am J Resp Cell Molec Biol 2000; 23(4): 546–54
Page K, Li J, Hodge JA, et al. Characterization of a Rac1 signaling pathway to cyclin D1 expression in airway smooth muscle cells. J Biol Chem 1999; 274: 22065–71
Page K, Li J, Wang Y, et al. Regulation of cyclin D1 expression and DNA synthesis by phosphatidylinositol 3-kinase in airway smooth muscle cells. Am J Resp Cell Molec Biol 2000; 23(4): 436–43
Krymskaya VP, Goncharova EA, Ammit AJ, et al. Src is necessary and sufficient for human airway smooth muscle cell proliferation and migration. FASEB J 2005; 19: 428–30
Krymskaya VP, Ammit AJ, Hoffman RK, et al. Activation of class IA phosphatidylinositol 3-kinase stimulates DNA synthesis in human airway smooth muscle cells. Am J Physiol 2001; 280: L1009–18
Roche S, Koegl M, Courtneidge SA. The phosphatidylinositol 3-kinase α is required for DNA synthesis induced by some, but not all, growth factors. Proc Natl Acad Sci U S A 1994; 91: 9185–9
Valius M, Kazlauskas A. Phospholipase C-γ1 and phosphatidylinositol 3 kinase are the downstream mediators of the PDGF receptor’s mitogenic signal. Cell 1993; 73: 321–34
McIlroy J, Chen D, Wjasow C, et al. Specific activation of p85-p110 phosphatidylinositol 3′-kinase stimulates DNA synthesis by ras- and p70 S6 kinase-dependent pathways. Mol Cell Biol 1997; 17: 248–55
Klippel A, Escobedo MA, Wachowicz MS, et al. Activation of phosphatidylinositol 3-kinase is sufficient for cell cycle entry and promotes cellular changes characteristic of oncogenic transformation. Mol Cell Biol 1998; 18: 5699–711
Weiss RH, Apostolids A. Dissociation of phosphatidylinositol 3-kinase activity and mitogenic inhibition in vascular smooth muscle cells. Cell Signal 1995; 7: 113–22
Saward L, Zahradka P. Angiotensin II activates phosphatidylinositol 3-kinase in vascular smooth muscle cells. Circ Res 1997; 81: 249–57
Bacqueville D, Casagrande F, Perret B, et al. Phosphatidylinositol 3-kinase inhibitors block aortic smooth muscle cell proliferation in mid-late G1 phase: effect on cyclin-dependent kinase 2 and the inhibitory protein p27KIP1. Biochem Biophys Res Commun 1998; 244: 630–6
Hu ZW, Shi XY, Hoffman BB. α1 Adrenergic receptors activate phosphatidylinositol 3-kinase in human vascular muscle cells. J Biol Chem 1996; 271: 8977–82
Takahashi T, Kawahara Y, Okuda M, et al. Angiotensin II stimulates mitogenactivated protein kinases and protein synthesis by a Ras-independent pathway in vascular smooth muscle cells. J Biol Chem 1997; 272: 16018–22
Carpenter CL, Cantley LC. Phosphoinositide 3-kinase and the regulation of cell growth. Biochim Biophys Acta 1996; 1288: M11–6
Panettieri RA, Hall IP, Maki CS, et al. α-Thrombin increases cytosolic calcium and induces human airway smooth muscle cell proliferation. Am J Resp Cell Molec Biol 1995; 13: 205–16
Thomas SM, Brugge JS. Cellular functions regulated by Src family kinases. Ann Rev Cell Develop Biol 1997; 13: 513–609
Brown MT, Cooper JA. Regulation, substrates and functions of Src. Biochim Biophys Acta 1996; 1287: 121–49
Tsang F, Hwa Choo H, Dawe GS, et al. Inhibitors of the tyrosine kinase signaling cascade attenuated thrombin-induced guinea pig airway smooth muscle cell proliferation. Biochem Biophys Res Commun 2002; 293(1): 72–8
Sayeski PP, Ali MS. The critical role of c-Src and the Shc/Grb2/ERK2 signaling pathway in angiotensin II-dependent VSMC proliferation. Exp Cell Res 2003; 287(2): 339–49
Luttrell LM, Ferguson SSG, Daaka Y, et al. β-Arrestin-dependent formation of β2 adrenergic receptor-Src protein kinase complexes. Science 1999; 283: 655–61
Marinissen MJ, Gutkind JS. G-protein-coupled receptors and signaling networks: emerging paradigms. Trends Pharmacol Sci 2001; 22(7): 368–76
Yano H, Nakanashi S, Kimura K, et al. Inhibition of histamine secretion by wortmannin through the blockade of phosphatidylinositol 3-kinase in RBL-2H3 cells. J Biol Chem 1993; 268: 25846–56
Bonser RW, Thompson NT, Randall RW, et al. Demethoxyviridin and wortmannin block phospholipase C and D activation in human neutrophil. Br J Pharmacol 1991; 103: 1237–41
Vemuri GS, Rittenhouse SE. Wortmannin inhibits serum-induced activation of phosphoinositide 3-kinase and proliferation of CHRF-288 cells. Biochem Biophys Res Commun 1994; 202: 1619–23
Kapeller R, Cantley LC. Phosphatidylinositol 3-kinase. BioEssays 1994; 16: 565–76
Hu Q, Klippel A, Muslin AJ, et al. Ras-dependent induction of cellular responses by constitutively active phosphatidylinositol-3 kinase. Science 1995; 268: 100–2
Klippel A, Reinhard C, Kavanaugh WM, et al. Membrane localization of phosphatidylinositol 3-kinase is sufficient to activate multiple signal-transducing kinase pathways. Mol Cell Biol 1996; 16: 4117–27
Chung J, Grammer TC, Lemon KP, et al. PDGF- and insulin-dependent pp70s6k activation mediated by phosphatidylinositol-3-OH kinase. Nature 1994; 370: 71–5
Lane HA, Fernandez A, Lamb NJC, et al. p70S6K function is essential for G1 progression. Nature 1993; 363: 170–2
Monfar M, Lemon KP, Grammer TC, et al. Activation of pp70/85 S6 kinases in interleukin-2-responsive lymphoid cells is mediated by phosphatidylinositol 3-kinase and inhibited by cyclic AMP. Mol Cell Biol 1995; 15: 326–37
Jefferies HBJ, Fumagalli S, Dennis PB, et al. Rapamycin suppresses 5′TOP mRNA translation through inhibition of p70s6k. EMBO J 1997; 16: 3693–704
Pullen N, Dennis PB, Andjelkovic M, et al. Phosphorylation and activation of p70s6k by PDK1. Science 1998; 279: 707–10
Alessi DR, Kozlowski MT, Weng Q, et al. 3-Phosphoinositide-dependent protein kinase 1 (PDK1) phosphorylates and activates the p70 S6 kinase in vivo and in vitro. Curr Biol 1997; 8: 69–81
Gosens R, Zaagsma J, Meurs H, et al. Muscarinic receptor signaling in the pathophysiology of asthma and COPD. Resp Res 2006; 9: 73
Giembycz MA. Phosphodiesterase-4: selective and dual-specificity inhibitors for the therapy of chronic obstructive pulmonary disease. Proc Am Thoracic Soc 2005; 2(4): 326–33
Spina D. The potential of PDE4 inhibitors in respiratory disease. Curr Drug Targets Inflam Allergy 2004; 3: 231–6
Vignola AM. PDE4 inhibitors in COPD: a more selective approach to treatment. Resp Med 2004; 98(6): 495–503
Leung SY, Eynott P, Nath P, et al. Effects of ciclesonide and fluticasone propionate on allergen-induced airway inflammation and remodeling features. J Allergy Clin Immunol 2005; 115(5): 989–96
Henderson WR Jr, Chiang GKS, Tien Y-T, et al. Reversal of allergen-induced airway remodeling by CysLT1 receptor blockade. Am J Resp Crit Care Med 2006; 173(7): 718–28
Stewart AG. Airway wall remodelling and hyperresponsiveness: modelling remodelling in vitro and in vivo. Pulmon Pharmacol Ther 2001; 14(3): 255–65
Strek ME. Difficult asthma. Proc Am Thoracic Soc 2006; 3(1): 116–23
Toews ML, Ediger TL, Romberger DJ, et al. Lysophosphatidic acid in airway function and disease. Biochim Biophys Acta 2002; 1582(1–3): 240–50
Hennessy BT, Smith DL, Ram PT, et al. Exploiting the PI3K/AKT/PKB pathway for cancer drug discovery. Nat Rev Drug Discov 2005; 4(12): 988–1004
Simon JA. Using isoform-specific inhibitors to target lipid kinases. Cell 2006; 125(4): 647–9
Bader AG, Kang S, Zhao L, et al. Oncogenic PI3K deregulates transcription and translation. Nat Rev Cancer 2005; 5(12): 921–9
Vivanco I, Sawyers CL. The phosphatidylinositol 3-kinase-Akt/PKB pathway in human cancer. Nat Rev Cancer 2002; 2: 489–501
Knight ZA, Gonzalez B, Feldman ME, et al. A pharmacological map of the PI3-K family defines a role for p110α in insulin signaling. Cell 2006; 125(4): 733–47
Foukas LC, Claret M, Pearce W, et al. Critical role for the p110α phosphoinositide-03-OH kinase in growth and metabolic regulation. Nature 2006; 441(7091): 366–70
Fan Q-W, Knight ZA, Goldenberg DD, et al. A dual PI3 kinase/mTOR inhibitor reveals emergent efficacy in glioma. Cancer Cell 2006; 9(5): 341–9
Camps M, Ruckle T, Ji H, et al. Blockade of PI3Kγ suppresses joint inflammation and damage in mouse models of rheumatoid arthritis. 2005; 11(9): 936–43
Barber DF, Bartolome A, Hernandez C, et al. PI3Kγ inhibition blocks glomerulo-nephritis and extends lifespan in a mouse model of systemic lupus. Nat Med 2005; 11(9): 933–5
Ohashi PS, Woodgett JR. Modulating autoimmunity: pick your PI3 kinase. Nat Med 2005; 11(9): 924–5
Hubbard SR. Protein tyrosine kinases: autoregulation and small-molecule inhibition. Curr Opin Struct Biol 2002; 12(6): 735–41
Bjornsti M-A, Houghton PJ. The TOR pathway: a target for cancer therapy. Nat Rev Cancer 2004; 4(5): 335–48
Zhou L, Goldsmith AM, Bentley JK, et al. 4E-Binding protein phosphorylation and eukaryotic initiation factor-4E release are required for airway smooth muscle hypertrophy. Am J Resp Cell Molec Biol 2005; 33(2): 195–202
Acknowledgments
This work was supported by grants from the National Institutes of Health, the LAM Foundation, and the American Lung Association.
The authors has no conflicts of interest that are directly relevant to the content of this review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Krymskaya, V.P. Targeting the Phosphatidylinositol 3-Kinase Pathway in Airway Smooth Muscle. BioDrugs 21, 85–95 (2007). https://doi.org/10.2165/00063030-200721020-00003
Published:
Issue Date:
DOI: https://doi.org/10.2165/00063030-200721020-00003