Abstract
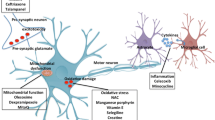
Amyotrophic lateral sclerosis (ALS) is a progressive neurodegenerative disease for which no cure or effective treatment presently exists. Many different types of drugs have been tested; most are based on various hypotheses of mechanisms for neuronal death, including oxidative damage, loss of trophic factor support, glutamate-mediated excitotoxicity, and chronic inflammation. The discovery that a small percentage of ALS cases are familial and involve mutation in a Superoxide dismutase gene (SOD1) led to the development of transgenic mouse models presently widely used for testing possible drugs. Mutations in the vascular endothelial growth factor gene (VEGF) also appear to be involved. Riluzole, an inhibitor of glutamate release and the only agent presently approved for clinical use, only extends survival by a few months. A number of trophic factors, anti-inflammatory agents, and inhibitors of oxidative stress have been reported to prolong survival in mouse models and some are now in clinical trials. Gene transfer of VEGF or glial cell-line derived neurotrophic factor, anti-inflammatory COX-2 inhibitors, and minocycline have had particularly promising results in mice. No breakthrough has yet occurred and present thinking is that combinations of drugs may be required to slow the multifactorial neurodegeneration process effectively.

Similar content being viewed by others
References
Strong M, Rosenfeld J. Amyotrophic lateral sclerosis: a review of current concepts. Amyotroph Lateral Scler Other Motor Neuron Disord 2003; 4: 136–43
Forshew DA, Bromberg MB. A survey of clinicians’ practice in the symptomatic treatment of ALS. Amyotroph Lateral Scler Other Motor Neuron Disord 2003; 4: 258–63
van den Berg LH, van denBerg JP, Mathus-Vliegen EM, et al. The symptomatic treatment of amyotrophic lateral sclerosis [in Dutch]. Ned Tijdschr Geneeskd 2004; 148(11): 513–8
Lambrechts D, Storkebaum E, Morimoto M, et al. VEGF is a modifier of amyotrophic lateral sclerosis in mice and humans and protects motoneurons against ischemic death. Nat Genet 2003; 34: 383–94
Oosthuyse B, Moons L, Storkebaum E, et al. Deletion of the hypoxia-response element in the vascular endothelial growth factor promoter causes motor neuron degeneration. Nat Genet 2001; 28: 131–8
McGeer EG, Olney JW, McGeer PL, editors. Kainic acid as a tool in neurobiology. New York: Raven Press, 1978
McGeer PL, McGeer EG, Scherer U, et al. A glutamatergic corticostriatal path? Brain Res 1977; 128: 369–73
Iwasaki Y, Ikeda K, Kinoshita M. Molecular and cellular mechanism of glutamate receptors in relation to amyotrophic lateral sclerosis. Curr Drug Targets CNS Neurol Disord 2002 Oct; 1(5): 511–8
Miller RG, Mitchell JD, Lyon M, et al. Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND). Amyotroph Lateral Scler Other Motor Neuron Disord 2003; 4(3): 191–206
Traynor BJ, Alexander M, Corr B, et al. An outcome study of riluzole in amyotrophic lateral sclerosis: a population-based study in Ireland, 1996-2000. J Neurol 2003; 250: 473–9
Lacomblez L, Bensimon G, Leigh PN, et al. Long-term safety of riluzole in amyotrophic lateral sclerosis: ALS Study Groups I and II. Amyotroph Lateral Scler Other Motor Neuron Disord 2002; 3(1): 23–9
Bensimon G, Lacomblez L, Delumeau JC, et al. A study of riluzole in the treatment of advanced stage or elderly patients with amyotrophic lateral sclerosis. J Neurol 2002; 249: 609–15
Ryberg H, Askmark H, Persson LI. A double-blind randomized clinical trial in amyotrophic lateral sclerosis using lamotrigine: effects on CSF glutamate, aspartate, branched-chain amino acid levels and clinical parameters. Acta Neurol Scand 2003; 108: 1–8
Miller RG, Moore II DH, Gelinas DF, et al. Phase III randomized trial of gabapentin in patients with amyotrophic lateral sclerosis. Neurology 2001; 56: 843–8
Kalra S, Cashman NR, Caramanos Z, et al. Gabapentin therapy for amyotrophic lateral sclerosis: lack of improvement in neuronal integrity shown by MR spectroscopy. Am J Neuroradiol 2003; 24: 476–80
Cudkowicz ME, Shefner JM, Schoenfeld DA, et al. A randomized, placebo-controlled trial of topiramate in amyotrophic lateral sclerosis. Neurology 2003; 61: 456–64
Snow RJ, Turnbull J, da Silva S, et al. Creatine supplementation and riluzole treatment provide similar beneficial effects in copper, zinc Superoxide dismutase (G93A) transgenic mice. Neuroscience 2003; 119: 661–7
Maragakis NJ, Jackson M, Ganel R, et al. Topiramate protects against motor neuron degeneration in organotypic spinal cord cultures but not in G93A SOD1 transgenic mice. Neurosci Lett 2003; 338: 107–10
Canton T, Bohme GA, Boireau A, et al. RPR 119990, a novel alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid antagonist: synthesis, pharmacological properties, and activity in an animal model of amyotrophic lateral sclerosis. J Pharmacol Exp Ther 2001; 299: 314–22
Andreassen OA, Dedeoglu A, Klivenyi P, et al. N-acetyl-L-cysteine improves survival and preserves motor performance in an animal model of familial amyotrophic lateral sclerosis. Neuroreport 2000; 11: 2491–3
Wu AS, Kiaei M, Aguirre N, et al. Iron porphyrin treatment extends survival in a transgenic animal model of amyotrophic lateral sclerosis. J Neurochem 2003; 85: 142–50
Nagano S, Fujii Y, Yamamoto T, et al. The efficacy of trientine or ascorbate alone compared to that of the combined treatment with these two agents in familial amyotrophic lateral sclerosis model mice. Exp Neurol 2003; 179: 176–80
Ferrante RJ, Klein AM, Dedeoglu A, et al. Therapeutic efficacy of EGb761 (Gingko biloba extract) in a transgenic mouse model of amyotrophic lateral sclerosis. J Mol Neurosci 2001; 17: 89–96
Jiang F, DeSilva S, Turnbull J. Beneficial effect of ginseng root in SOD-1 (G93A) transgenic mice. J Neurol Sci 2000; 180: 52–4
Poduslo JF, Whelan SL, Curran GL, et al. Therapeutic benefit of polyamine-modified catalase as a scavenger of hydrogen peroxide and nitric oxide in familial amyotrophic lateral sclerosis transgenics. Ann Neurol 2000; 48: 943–7
Turner BJ, Cheah IK, Macfarlane KJ, et al. Antisense peptide nucleic acid-mediated knockdown of the p75 neurotrophin receptor delays motor neuron disease in mutant SOD1 transgenic mice. J Neurochem 2003; 87: 752–63
Kaspar BK, Llado J, Sherkat N, et al. Retrograde viral delivery of IGF-1 prolongs survival in a mouse ALS model. Science 2003; 301: 839–42
Manabe Y, Nagano I, Gazi MS, et al. Glial cell line-derived neurotrophic factor protein prevents motor neuron loss of transgenic model mice for amyotrophic lateral sclerosis. Neurol Res 2003; 25: 195–200
Manabe Y, Nagano I, Gazi MS, et al. Adenovirus-mediated gene transfer of glial cell line-derived neurotrophic factor prevents motor neuron loss of transgenic model mice for amyotrophic lateral sclerosis. Apoptosis 2002; 7: 329–34
Wang LJ, Lu YY, Muramatsu S, et al. Neuroprotective effects of glial cell line-derived neurotrophic factor mediated by an adeno-associated virus vector in a transgenic animal model of amyotrophic lateral sclerosis. J Neurosci 2002; 22: 6920–8
Acsadi G, Anguelov RA, Yang H, et al. Increased survival and function of SOD1 mice after glial cell-derived neurotrophic factor gene therapy. Hum Gene Ther 2002; 13: 1047–59
Azzouz M, Ralph GS, Storkebaum E, et al. VEGF delivery with retrogradely transported lentivector prolongs survival in a mouse ALS model. Nature 2004; 429: 413–7
Feeney SJ, Austin L, Bennett TM, et al. The effect of leukaemia inhibitory factor on SOD1 G93A murine amyotrophic lateral sclerosis. Cytokine 2003; 23: 108–18
Azari MF, Galle A, Lopes EC, et al. Leukemia inhibitory factor by systemic administration rescues spinal motor neurons in the SOD1 G93A murine model of familial amyotrophic lateral sclerosis. Brain Res 2001; 922: 144–7
Bordet T, Lesbordes JC, Rouhani S, et al. Protective effects of cardiotrophin-1 adenoviral gene transfer on neuromuscular degeneration in transgenic ALS mice. Hum Mol Gen 2001; 10: 1925–33
Klivenyi P, Kiaei M, Gardian G, et al. Additive neuroprotective effects of creatine and cyclooxygenase 2 inhibitors in a transgenic mouse model of amyotrophic lateral sclerosis. J Neurochem 2004; 88: 576–82
Derave W, Van Den Bosch L, Lemmens G, et al. Skeletal muscle properties in a transgenic mouse model for amyotrophic lateral sclerosis: effects of creatine treatment. Neurobiol Dis 2003; 13: 264–72
Groeneveld GJ, Veldink JH, van der Tweel I, et al. A randomized sequential trial of creatine in amyotrophic lateral sclerosis. Ann Neurol 2003; 53: 437–45
Pompl PN, Ho L, Bianchi M, et al. A therapeutic role for cyclooxygenase-2 inhibitors in a transgenic mouse model of amyotrophic lateral sclerosis. FASEB J 2003; 17: 725–7
Drachman DB, Frank K, Dykes-Hoberg M, et al. Cyclooxygenase 2 inhibition protects motor neurons and prolongs survival in a transgenic mouse model of ALS. Ann Neurol 2002; 52: 771–8
Kriz J, Nguyen MD, Julien JP. Minocycline slows disease progression in a mouse model of amyotrophic lateral sclerosis. Neurobiol Dis 2002; 10: 268–78
Van Den Bosch L, Tilkin P, Lemmens G, et al. Minocycline delays disease onset and mortality in a transgenic model of ALS. Neuroreport 2002; 13: 1067–70
Zhang W, Narayanan M, Friedlander RM. Additive neuroprotective effects of minocycline with creatine in a mouse model of ALS. Ann Neurol 2003; 53: 267–70
Anneser JM, Gmerek A, Gerkrath J, et al. Immunosuppressant FK506 does not exert beneficial effects in symptomatic G93A Superoxide dismutase-1 transgenic mice. Neuroreport 2001; 12: 2663–5
Kieran D, Kalmar B, Dick JR, et al. Treatment with arimoclomol, a coinducer of heat shock proteins, delays disease progression in ALS mice. Nat Med 2004; 10: 402–5
Kirkinezos IG, Hernandez D, Bradley WG, et al. An ALS mouse model with a permeable blood-brain barrier benefits from systemic cyclosporine A treatment. J Neurochem 2004; 88: 821–6
Karlsson J, Fong KS, Hansson MJ, et al. Life span extension and reduced neuronal death after weekly intraventricular cyclosporin injections in the G93 A transgenic nare mouse model of amyotrophic lateral sclerosis. J Neurosurg 2004; 101: 128–37
Turner BJ, Lopes EC, Cheema SS. The serotonin precursor 5-hydroxytryptophan delays neuromuscular disease in murine familial amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord 2003; 4: 171–6
Turner BJ, Rembach A, Spark R, et al. Opposing effects of low and high-dose clozapine on survival of transgenic amyotrophic lateral sclerosis mice. J Neurosci Res 2003; 74: 605–13
Raman C, McAllister SD, Rizvi G, et al. Amyotrophic lateral sclerosis: delayed disease progression in mice by treatment with a cannabinoid. Amyotroph Lateral Scler Other Motor Neuron Disord 2004; 5: 33–9
Valerio A, Ferrario M, Paterlini M, et al. Spinal cord mGlu1a receptors: possible target for amyotrophic lateral sclerosis therapy. Pharmacol Biochem Behav 2002; 73: 447–54
DiMatteo V, Esposito E. Biochemical and therapeutic effects of antioxidants in the treatment of Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis. Curr Drug Targets CNS Neurol Disord 2003; 2: 95–107
Butterfield DA, Kanski J. Brain protein oxidation in age-related neurodegenerative disorders that are associated with aggregated proteins. Mech Ageing Dev 2001; 122: 945–62
Cleveland DW, Rothstein JD. From Charcot to Lou Gehrig: deciphering selective motor neuron death in ALS. Nat Rev Neurosci 2001; 2: 806–19
Pioro EP. Antioxidant therapy in ALS. Amyotroph Lateral Scler Other Motor Neuron Disord 2000; 1 Suppl. 4: 5–15
Beal MF, Ferrante RJ, Browne SE, et al. Increased 3-nitrotyrosine in both sporadic and familial amyotrophic lateral sclerosis. Ann Neurol 1997; 42: 646–54
Ferrante RJ, Browne SE, Shinobu LA, et al. Evidence of increased oxidative damage in both sporadic and familial amyotrophic lateral sclerosis. J Neurochem 1997; 69: 2064–74
Anneser JMH, Cookson MR, Ince PG, et al. Glial cells of the spinal cord and subcortical white matter upregulate nitric oxide synthase in sporadic amyotrophic lateral sclerosis. Exp Neurol 2001; 171: 418–21
Pedersen WA, Fu W, Keller JN, et al. Protein modification by the lipid peroxidation product 4-hydroxynonenal in the spinal cords of amyotrophic lateral sclerosis. Ann Neurol 1998; 44: 8819–24
Chous SM, Han CY, Wang HS, et al. A receptor for advanced glycosylation endproducts (AGEs) is colocalized with neurofilament-bound AGEs and SOD1 in motoneurons of ALS: immunohistochemical study. J Neurol Sci 1999; 169: 87–92
Shibata N, Nagai R, Uchida K, et al. Morphological evidence for lipid peroxidation and protein glycoxidation in spinal cords from sporadic amyotrophic lateral sclerosis patients. Brain Res 2001; 917: 97–104
Carter GT, Krivickas LS, Weydt P, et al. Drug therapy for amyotrophic lateral sclerosis: where are we now? IDrugs 2003; 6: 147–53
Kwiecinski H, Janik P, Jamrozik Z, et al. The effect of selegiline and vitamin E in the treatment of ALS: an open randomized clinical trials [in Polish]. Neurol Neurochir Pol 2001; 35(1 Suppl.): 101–6
Desnuelle C, Dib M, Garrel C, et al. A double-blind, placebo-controlled randomized clinical trial of alpha-tocopherol (vitamin E) in the treatment of amyotrophic lateral sclerosis (ALS). Amyotroph Lateral Scler Other Motor Neuron Disord 2001; 2: 9–18
Louwerse ES, Weverling GJ, Bossuyt PM, et al. Randomized double-blind controlled trial of acetylcysteine in amyotrophic lateral sclerosis. Arch Neurol 1995; 52: 559–64
Vyth A, Timmer JG, Bossuyt PM, et al. Survival in patients with amyotrophic lateral sclerosis treated with an array of antioxidants. J Neurol Sci 1996; 139 Suppl.: 99–103
Pattee GL, Post GR, Gerber RE, et al. Reduction of oxidative stress in amyotrophic lateral sclerosis following pramipexole treatment. Amyotroph Lateral Scler Other Motor Neuron Disord 2003; 4: 90–5
Li B, Xu W, Luo C, et al. VEGF-induced activation of the PI3-K/Akt pathway reduces mutant SOD1-mediated motor neuron cell death. Brain Res Mol Brain Res 2003; 111(1-2): 155–64
Kalra S, Genge A, Arnold DL. A prospective, randomized, placebo-controlled evaluation of corticoneuronal response to intrathecal BDNF therapy in ALS using magnetic resonance spectroscopy: feasibility and results. Amyotroph Lateral Scler Other Motor Neuron Disord 2003; 4: 22–6
Ochs G, Penn RD, York M, et al. A phase I/II trial of recombinant methionyl human brain derived neurotrophic factor administered by intrathecal infusion to patients with amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord 2000; 1: 201–6
Mitchell JD, Wokke JH, Borasio GD. Recombinant human insulin-like growth factor I (rhIGF-I) for amyotrophic lateral sclerosis/motor neuron disease. Cochrane Database Syst Rev 2002; (3): CD002064
Xaliproden: SR 57746, SR 57746A, xaliproden hydrochloride, xaliprodene. Drugs R D 2003; 4: 386-8
Meininger V, Bensimon G, Bradley WR, et al. Efficacy and safety of xaliproden in amyotrophic lateral sclerosis: results of two phase III trials. Amyotroph Lateral Scler Other Motor Neuron Disord 2004; 5: 107–17
Lacomblez L, Bensimon G, Douillet P, et al. Xaliproden in amyotrophic lateral sclerosis: early clinical trials. Amyotroph Lateral Scler Other Motor Neuron Disord 2004; 5: 99–106
McGeer PL, McGeer EG. Inflammatory processes in amyotrophic lateral sclerosis. Muscle Nerve 2002; 26: 459–70
Alexianu ME, Kozovska M, Appel SH. Immune reactivity in a mouse model of familial ALS correlates with disease progression. Neurology 2001; 57: 1282–9
Yasojima K, Tourtellotte WW, McGeer EG, et al. Marked increase in cyclooxygenase-2 in ALS spinal cord. Neurology 2001; 57: 952–6
Giulian D, Haverkamp LJ, Yu JH, et al. Specific domains of β-amyloid from Alzheimer plaque elicit neuron killing in human microglia. J Neurosci 1996; 16: 6021–37
Giulian D, Vaca K, Noonan CA. Secretion of neurotoxins by mononuclear phagocytes infected with HIV-1. Science 1990; 250: 1593–6
Klegeris A, McGeer PL. Rat brain microglia and peritoneal macrophages show similar responses to respiratory burst stimulants. J Neuroimmunol 1994; 53: 83–90
Colton CA, Gilbert DI. Production of Superoxide anions by a CNS macrophage, the microglia. FEBS Lett 1987; 223: 284–8
Klegeris A, Walker DG, McGeer PL. Regulation of glutamate in cultures of human monocytic THP-1 and astrocytoma U-373 MG cells. J Neuroimmunol 1997; 78: 152–61
Weydt P, Weiss MD, Moller T, et al. Neuro-inflammation as a therapeutic target in amyotrophic lateral sclerosis. Curr Opin Investig Drugs 2002; 3(12): 1720–4
McGeer PL. COX-2 and ALS. Amyotroph Lateral Scler Other Motor Disord 2001; 2: 121–2
Aimer G, Guegan C, Teismann P, et al. Increased expression of the proinflammatory enzyme cyclooxygenase-2 in amyotrophic lateral sclerosis. Ann Neurol 2001; 49: 176–85
Drachman DB, Rothstein JD. Inhibition of cyclooxygenase-2 protects motor neurons in an organotypic model of amyotrophic lateral sclerosis. Ann Neurol 2000; 48: 792–5
Barneoud P, Curet O. Beneficial effects of lysine acetylsalicylate, a soluble salt of aspirin, on motor performance in a transgenic model of amyotrophic lateral sclerosis. Exp Neurol 1999; 155: 243–51
Ladecola C, Niwa K, Nogawa S, et al. Reduced susceptibility to ischemic brain injury and N-methyl-D-aspartate neurotoxicity in cyclooxygenase-2-deficient mice. Proc Natl Acad Sci U S A 2001; 98: 1294–9
Amtmann D, Weydt P, Johnson KL, et al. Survey of cannabis use in patients with amyotrophic lateral sclerosis. Am J Hosp Palliat Care 2004; 21: 95–104
Carter GT, Rosen BS. Marijuana in the management of amyotrophic lateral sclerosis. Am J Hosp Palliat Care 2001; 18: 264–70
Klegeris A, Bissonnette CJ, McGeer PL. Reduction of human monocytic cell neurotoxicity and cytokine excretion by ligands of the cannabinoid-type CB2 receptor. Br J Pharmacol 2003; 139: 775–86
Jacob S, Poeggeler B, Weishaupt JH, et al. Melatonin as a candidate compound for neuroprotection in amyotrophic lateral sclerosis (ALS): high tolerability of daily oral melatonin administration in ALS patients. J Pineal Res 2002; 33: 186–7
Parton M, Mitsumoto H, Leigh PN. Amino acids for amyotrophic lateral sclerosis/ motor neuron disease. Cochrane Database Syst Rev 2003; (4): CD003457
Kriz J, Gowing G, Julien JP. Efficient three-drug cocktail for disease induced by mutant Superoxide dismutase. Ann Neurol 2003; 53: 429–36
Acknowledgments
Supported by the Jack Brown and Family AD Research Fund, the George Hodgson bequest, Alzheimer Society of Canada, Astra Zeneca, and individual British Columbians.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
McGeer, E.G., McGeer, P.L. Pharmacologic Approaches to the Treatment of Amyotrophic Lateral Sclerosis. BioDrugs 19, 31–37 (2005). https://doi.org/10.2165/00063030-200519010-00004
Published:
Issue Date:
DOI: https://doi.org/10.2165/00063030-200519010-00004