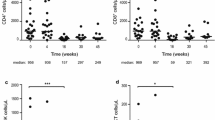
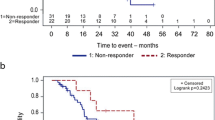
Abstract
Alemtuzumab is a humanized therapeutic monoclonal antibody (MAb) that recognizes the CD52 antigen, expressed on normal and neoplastic lymphocytes, monocytes, and natural killer cells. In 2001, alemtuzumab was approved in the US and Europe to treat B-cell chronic lymphocytic leukemia (CLL) that had been treated previously with alkylating agents and was refractory to fludarabine. In heavily pretreated patients this MAb is able to produce response rates of about 40%, and in symptomatic, previously untreated patients response rates of more than 80% can be achieved. Alemtuzumab can also be used in patients with CLL as a preparative regimen for stem cell transplantation (SCT) and to prevent graft versus host disease. Moreover its in vivo use before or after SCT may also potentially result in depletion of residual leukemia cells, especially in the autologous setting. Adverse events associated with alemtuzumab include acute first-dose reaction, hematologic toxicity, and infectious complications. Usually they are predictable, manageable, and acceptable in the context of CLL. However, in a significant percentage of patients, cytomegalovirus reactivation occurs during alemtuzumab therapy, and routine weekly monitoring with the polymerase chain reaction methodology is indicated. Moreover, antiviral and antibacterial prophylaxis is mandatory.



Similar content being viewed by others
References
Rozman C, Montserrat E. Chronic lymphocytic leukemia. N Engl J Med 1995; 333: 1052–7
Catovsky D, Murphy RL. Key issues in the treatment of chronic lymphocytic leukemia (CLL). Eur J Cancer 1995; 31A: 2146–54
O’Brien S, delGiglio A, Keating M. Advances in biology and treatment of B-cell chronic lymphocytic leukemia. Blood 1995; 85: 307–18
Rai KR, Sawitsky A, Cronkite EP, et al. Clinical staging of chronic lymphocytic leukemia. Blood 1975; 46: 219–34
Robak T, Kasznicki M. Alkylating agents and nucleoside analogues in the treatment of B cell chronic lymphocytic leukemia. Leukemia 2002; 16: 1015–27
Jaksic B, Brugiatelli M, Krc I, et al. High dose chlorambucil versus Binet’s modified cyclophosphamide, doxorubicin, vincristine and prednisone regimen in the treatment of patients with advanced B-cell chronic lymphocytic leukemia: results of an international multicenter randomized trial. International Society for Chemo-Immunotherapy, Vienna. Cancer 1997; 79: 2107–19
Rai KR, Peterson BL, Appelbaum FR, et al. Fludarabine compared with chlorambucil as primary therapy for chronic lymphocytic leukemia. N Engl J Med 2000; 343: 1750–7
Robak T, Blonski JZ, Kasznicki M, et al. Cladribine with prednisone versus chlorambucil with prednisone as first-line therapy in chronic lymphocytic leukemia: report of a prospective, randomized, multicenter trial. Blood 2000; 96: 2723–9
Lin TS, Lucas MS, Byrd JC. Rituximab in B-cell chronic lymphocytic leukemia. Semin Oncol 2003; 30: 483–92
Moreton P, Hillmen P. Alemtuzumab therapy in B-cell lymphoproliferative disorders. Semin Oncol 2003; 30: 493–501
Robak T. Monoclonal antibodies in the treatment of chronic lymphoid leukemias. Leuk Lymphoma 2004; 45: 205–19
Osterborg A, Dyer MJ, Bunjes D, et al. Phase II multicenter study of human CD52 antibody in previously treated chronic lymphocytic leukemia. J Clin Oncol 1997; 15: 1567–74
Rai KR, Freter CE, Mercier RJ, et al. Alemtuzumab in previously treated chronic lymphocytic leukemia patients who also had received fludarabine. J Clin Oncol 2002; 20: 3891–7
Lundin J, Kimby E, Bjorkholm M, et al. Phase II trial of subcutaneous anti CD52 monoclonal antibody alemtuzumab (Campath-1H) as first-line treatment for patients with B-cell chronic lymphocytic leukemia (B-CLL). Blood 2002; 100: 768–73
Keating MJ, Flinn I, Jain V, et al. Therapeutic role of alemtuzumab (Campath-1H) in patients who have failed fludarabine: results of a large international study. Blood 2002; 99: 3554–61
Rebello PR, Hale G, Friend PJ, et al. Anti-globulin responses to rat and humanized CAMPATH-1 monoclonal antibody used to treat transplant rejection. Transplantation 1999; 68: 1417–20
Villamor N, Montserrat E, Colomer D. Mechanism of action and resistance to monoclonal antibody therapy. Semin Oncol 2003; 30: 424–33
Hale G, Bright S, Chumbley G, et al. Removal of T cells from bone marrow for transplantation: a monoclonal antilymphocyte antibody that fixes human complement. Blood 1983; 62: 873–83
Treumann A, Lifely MR, Schneider P, et al. Primary structure of CD52. J Biol Chem 1995; 270: 6088–99
Hederer RA, Guntermann C, Miller N, et al. The CD45 tyrosine phosphatase regulates Campath-1H (CD52): induced TCR dependent signal transduction in human T cells. Int Immunol 2000; 12: 505–16
Hale C, Bartholomew M, Taylor V. Recognition of CD52 allelic gene products by CAMPATH-1H antibodies. Immunology 1996; 88: 183–90
Ginaldi L, De Martinis M, Matutes E, et al. Levels of expression of CD52 in normal and leukemic B and T cells: correlations with in vivo therapeutic responses to CAMPATH-1H. Leuk Res 1998; 22: 185–91
Quigley MM, Bethel KJ, Sharpe RW, et al. CD52 expression in hairy cell leukemia. Am J Hematol 2003; 74: 227–30
Giles FJ, Vose JM, Do KA, et al. Circulating CD20 and CD52 in patients with non-Hodgkin’s lymphoma or Hodgkin’s disease. Br J Haematol 2003; 123: 850–7
Albitar M, Do KA, Johnson MM, et al. Free circulating soluble CD52 as a tumor marker in chronic lymphocytic leukemia and its implication in therapy with anti-CD52 antibodies. Cancer 2004; 101: 999–8
Xia MQ, Hale G, Waldmann H. Efficient complement mediated lysis of cells containing the CAMPATH-1 (CD w52) antigen. Mol Immunol 1993; 30: 1089–96
Frampton JE, Wagstaff AJ. Alemtuzumab. Drugs 2003; 63: 1229–43
Hale G. The CD52 antigen and development of the CAMPATH antibodies. Cytotherapy 2002; 3: 137–43
Stanglmaier M, Reis S, Hallek M. Rituximab and alemtuzumab induce a nonclassic, caspase-independent apoptotic pathway in B-lymphoid cell lines and in chronic lymphocytic leukemia cells. Ann Hematol 2004; 83: 634–45
Zent CS, Chen JB, Kurten RC, et al. Alemtuzumab (CAMPATH-1H) does not kill chronic lymphocytic leukemia cells in serum free medium. Leuk Res 2004; 28: 495–7
Smolewski P, Darzynkiewicz Z, Robak T. Caspase mediated cell death in hematological malignancies: theoretical considerations, methods of assessment and clinical implications. Leuk Lymphoma 2003; 44: 1089–104
Smolewski P, Szmigielska-Kaplon A, Cebula B, et al. Molecular mechanisms of pro-apoptotic activity of alemtuzumab: potential prognostic implications and the rationale for combined therapy in B-cell chronic lymphocytic leukemia patients [abstract 3442]. Blood 2003; 102 Suppl. 1: 925a
Clynes RA, Towers TL, Presta LG, et al. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat Med 2000; 6: 443–6
Zhang Z, Zhang M, Goldman CK, et al. Effective therapy for a murine model of adult T-cell leukemia with the humanized anti-CD52 monoclonal antibody. Campath-1H. Cancer Res 2003; 63: 6453–7
Cartron G, Dacheux L, Salles G, et al. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor Fcgam-maRIIIa gene. Blood 2002; 99: 754–8
Lin TS, Flinn IW, Modali R, et al. FCGR3A and FCGR2A polymorphisms may not correlate with response to alemtuzumab (Campath-1H) in chronic lymphocytic leukemia (CLL). Blood Epub 2004 Jun 24
Flynn JM, Byrd JC. Campath-1H monoclonal antibody therapy. Curr Opin Oncol 2000; 12: 574–81
Isaacs JD, Watts RA, Hazleman BL, et al. Humanised monoclonal antibody therapy for rheumatoid arthritis. Lancet 1992; 340: 748–52
Tang SC, Hewitt K, Reis MD, et al. Immunosuppressive toxicity of CAMPATH-1 H monoclonal antibody in the patients with recurrent low grade lymphoma. Leuk Lymphoma 1996; 24: 93–101
Rebello P, Hale G. Pharmacokinetics of CAMPATH-1H: assay development and validation. J Immunol Methods 2002; 260: 285–302
Nabhan C, Dyer MJ, Rosen ST. Current status of monoclonal antibody therapy for chronic lymphocytic leukemia. Oncology 2003; 17: 253–62
Montillo M, Cafro AM, Tedeschi A, et al. Safety and efficacy of subcutaneous Campath-1H for treating residual disease in patients with chronic lymphocytic leukemia responding to fludarabine. Haematologica 2002; 87: 695–700
O’Brien SM, Kantarjian HM, Thomas DA, et al. Alemtuzumab as treatment for residual disease after chemotherapy in patients with chronic lymphocytic leukemia. Cancer 2003 Dec 15; 98(12): 2657–63
Hale G, Rebello P, Kimby E, et al. Blood levels of alemtuzumab during treatment of patients with chronic lymphocytic leukemia: comparison of intravenous and subcutaneous route of administration [abstract]. Blood 2002; 100 Suppl. 1: 777
Tallman MS. Monoclonal antibody therapies in leukemias. Semin Hematol 2002; 39(4 Suppl. 3): 12–9
Hale G, Rebello P, Brettmau L, et al. Blood concentrations of alemtuzumab and antiglobulin responses in patients with chronic lymphocytic leukemia following intravenous or subcutaneous routes of administration. Blood 2004; 104: 948–55
Rossmann ED, Lundin R, Lenkei R, et al. Variability in B-cell antigen expression: implications for the treatment of B-cell lymphomas and leukemias with monoclonal antibodies. Hematol J 2001; 2: 300–6
Bowen AL, Zomas A, Emmett E, et al. Subcutaneous CAMPATH-1 H in fludarabine resistant/relapsed chronic lymphocytic and B-prolymphocytic leukaemia. Br J Haematol 1997; 96: 617–9
Ferrajoli A, O’Brien SM, Cortes E, et al. Phase II study of alemtuzumab in chronic lymphoproliferative disorders. Cancer 2003; 98: 773–8
Kennedy B, Rawstron A, Haynes A, et al. Eradication of detectable minimal disease with Campath-1H therapy results in prolonged survival in patients with refractory B-CLL [abstract 1544]. Blood 2001; 98: 367a
Lozanski G, Heerema NA, Flinn IW, et al. Alemtuzumab is an effective therapy for chronic lymphocytic leukemia with p53 mutations and deletions. Blood 2004; 103: 3278–81
McCune SL, Gockerman JP, Moore JO, et al. Alemtuzumab in relapsed or refractory chronic lymphocytic leukemia and prolymphocytic leukaemia. Leuk Lymphoma 2002; 43: 1007–11
Rawstron AC, Kennedy B, Moreton P, et al. Early prediction of outcome and response to alemtuzumab therapy in chronic lymphocytic leukemia. Blood 2004; 103: 2027–31
Kennedy B, Rawstron A, Carter C, et al. Campath-1H and fludarabine in combination are highly active in refractory chronic lymphocytic leukaemia. Blood 2002; 99: 2245–7
Faderl S, Thomas DA, O’Brien S, et al. Experience with alemtuzumab plus rituximab in patients with relapsed and refractory lymphoid malignancies. Blood 2003; 101: 3413–5
Nabhan C, Tallman MS, Riley MB, et al. Phase I study of combining rituximab and Campath-1H in patients with relapsed or refractory chronic lymphocytic leukemia [abstract 1536]. Blood 2001; 98 Suppl. 1: 365a
Stilgenbauer S, Dohner H. Campath-1H: induced complete remission of chronic lymphocytic leukemia despite p53 gene mutation and resistance to chemotherapy. N Engl J Med 2002; 347: 452–3
Cheson BD, Bennett JM, Grever M, et al. National Cancer Institute sponsored Working Group guidelines for chronic lymphocytic leukemia: revised guidelines of diagnosis and treatment. Blood 1996; 87: 4990–7
Khorana A, Bunn P, McLaughlin P, et al. A phase II multicenter study of CAMPATH-1 H antibody in previously treated patients with nonbulky non-Hodgkin’s lymphoma. Leuk Lymphoma 2001; 41: 77–87
Lundin J, Osterborg A, Brittinger D, et al. CAMPATH-1H monoclonal antibody in therapy for previously treated low-grade non-Hodgkin’s lymphoma: a phase II multicenter study. J Clin Oncol 1998; 16: 3257–63
Enblad G, Hagberg H, Erlanson B, et al. A pilot study of alemtuzumab (anti-CD52 monoclonal antibody) therapy for patients with relapsed or chemotherapy refractory peripheral T-cell lymphomas. Blood 2004; 103: 2920–4
Dohner H, Fischer K, Bentz M, et al. P53 gene deletion predicts for poor survival and non-response to therapy with purine analogs in chronic B-cell leukemias. Blood 1995; 85: 158–9
Wattel E, Preudhomme C, Hecquet B, et al. P53 mutations are associated with resistance to chemotherapy and short survival in hematologic malignancies. Blood 1994; 84: 3148–57
elRouby S, Thomas A, Costin D, et al. P53 gene mutation in B-cell chronic lymphocytic leukemia is associated with drug resistance and is independent of MDR1/MDR3 gene expression. Blood 1993; 82: 3452–9
Byrd JC, Smith L, Hackbarth ML, et al. Interphase cytogenetic abnormalities in chronic lymphocytic leukemia may predict response to rituximab. Cancer Res 2003; 63: 36–8
Pangalis GA, Dimopoulou MN, Angelopoulou MK, et al. Campath-1H in B-chronic lymphocytic leukemia: report on a patient treated thrice in a 3 year period. Med Oncol 2000; 17: 70–3
Rawstron AC, Kennedy B, Evans PA, et al. Quantitation of minimal disease levels in chronic lymphocytic leukemia using a sensitive flow cytometric assay improves the prediction of outcome and can be used to optimize therapy. Blood 2001; 98: 29–35
Osterborg A, Fassas AS, Anagnostopoulos A, et al. Humanized CD52 monoclonal antibody Campath-1H as first line treatment in chronic lymphocytic leukemia. Br J Haematol 1996; 93: 151–3
Dumont FJ. CAMPATH (alemtuzumab) for the treatment of chronic lymphocytic leukemia and beyond. Expert Rev Anticancer Ther 2002; 2: 23–35
Skotnicki AB, Robak T, Mayer J, et al. Phase III study to evaluate the efficacy and safety of front-line therapy with alemtuzumab (Campath®, MabCampath®) vs chlorambucil in patients with progressive B-cell chronic lymphocytic leukemia (CAM 307) [abstract 1599]. Blood 2003; 102 Suppl. 1: 439a
Robak T, Skotnicki AB, Mayer J, et al. Interim safety summary of alemtuzumab (CAMPATH®, MABCAMPATH®) vs chlorambucil as front-line therapy for patients with progressive B-cell chronic lymphocytic leukemia (B-CLL) [abstract 6563]. Proc Am Soc Clin Oncol Meeting 2004; 23: 571
Elter T, Borchmann P, Reiser M, et al. Development of a new, four-weekly schedule (FluCam) with concomitant application of Campath-1H and fludarabine in patients with relapsed/refractory CLL. Proc Am Soc Clin Oncol Meeting 2003; 22: 580
Rai KR, Byrd JC, Peterson BL, et al. A phase II trial of fludarabine followed by alemtuzumab (Campath-1H) in previously untreated chronic lymphocytic leukemia (CLL) patients with active disease: Cancer and Leukemia Group B (CALGB) Study 199 [abstract 772]. Blood 2002; 100 Suppl. 1: 205a
Dyer MJ, Kelsey SM, Mackay HJ, et al. In vivo purging of residual disease in CLL with Campath-1H. Br J Haematol 1997; 97: 669–72
Wendtner CM, Ritgen M, Schweighofer CD, et al. Consolidation with alemtuzumab in patients with chronic lymphocytic leukemia (CLL) in first remission: experience on safety and efficacy within a randomized multicenter phase III trial of the German CLL Study Group (GCLLSG). Leukemia 2004; 18: 1093–101
Nabhan C, Rosen ST. Conceptual aspects of combining rituximab and Campath-1H in the treatment of chronic lymphocytic leukemia. Semin Oncol 2002; 29 Suppl. 2: 75–80
Pawson R, Dyer MJS, Barge R, et al. Treatment of T-cell prolymphocytic leukemia with human CD52 antibody. J Clin Oncol 1997; 15: 2667–73
Keating MJ, Cazin B, Coutre S, et al. Campath-1H treatment of T-cell prolymphocytic leukemia in patients for whom at least one prior chemotherapy regimen has failed. J Clin Oncol 2002; 20: 205–13
Rosenblum MD, LaBelle J, Chang CC, et al. Efficacy of alemtuzumab treatment for refractory T-cell large granular lymphocytic leukemia. Blood 2004; 103: 1969–71
Hale G, Jacobs P, Wood L, et al. CD52 antibodies for prevention of graft versus host disease and graft rejection following transplantation of allogenic peripheral blood stem cells. Bone Marrow Transplant 2000; 26: 69–76
Hale G. Alemtuzumab in stem cell transplantation. Med Oncol 2002; 19 Suppl. 1: S33–47
Hale G, Slavin S, Goldman JM, et al. Alemtuzumab (Campath-1H) for treatment of lymphoid malignancies in the age of nonmyeloablative conditioning. Bone Marrow Transplant 2002; 30: 797–804
Vasil T, Rai KR, Mehrotra B. The role of monoclonal antibodies in stem cell transplantation. Semin Oncol 2004; 31: 83–9
Montillo M, Tedeschi A, Rossi V, et al. Successful CD34+ cell mobilization by intermediate-dose Ara-C in chronic lymphocytic leukemia patients treated with sequential fludarabine and Campath-1H. Leukemia 2004; 18: 57–62
Kennedy B, Rawstron AC, Evans P. Campath-1H therapy in 29 patients with refractory CLL ‘true’ complete remission is an attainable goal [abstract 2683]. Blood 1999; 94 Suppl. 1: 603a
Kottaridis PD, Miligan DW, Chopra R, et al. In vivo CAMPATH-1H prevents graft versus host disease following nonmyeloablative stem cell transplantation. Blood 2000; 96: 2419–25
Lush RJ, Haynes AP, Byrne J, et al. Allogenic stem-cell transplantation for lymphoproliferative disorders using BEAM-CAMPATH (+/- fludarabine) conditioning combined with post-transplant donor lymphocyte infusion. Cytotherapy 2001;3: 210–8
Faulkner RD, Craddock C, Byrne JL, et al. BEAM: alemtuzumab reduced intensity allogenic stem cell transplantation for lymphoproliferative disease. GVHD, toxicity and survival in 65 patients. Blood 2004; 103: 428–34
Peggs KS. Role of MabCampath in allogenic transplantation. Ann Hematol 2004; 89 Suppl. 1: S66–68
Carella AM, Beltrami G, Scalzulli PR, et al. Alemtuzumab can successfully treat steroid-refractory acute graft-versus-host disease (a GVHD). Bone Marrow Transplant 2004; 33: 131–2
Wolf D, Steiner B, Stilgeubauer S, et al. Treatment with Campath-1H for relapsed chronic lymphocytic leukemia after allogenic peripheral blood stem cell transplantation does not abrogate the development of chronic GVHD. Eur J Haematol 2004; 72: 145–8
Wing MG, Moreau T, Greenwood J, et al. Mechanism of first-dose cytokine release syndrome by Campath-1H: involvement of CD16 (Fcgamma RIII) and CD1 la/ CD18 (LFA-1) on NK cells. J Clin Invest 1996; 98: 2819–26
Keating M, Coutre S, Rai K, et al. Management guidelines for use of alemtuzumab in B-cell chronic lymphocytic leukemia. Clin Lymphoma 2004; 4: 220–7
Lundin J, Porwit-MacDonald A, Rossmann ED, et al. Cellular immune reconstitution after subcutaneous alemtuzumab (anti-CD52 monoclonal antibody, CAMPATH-1H) treatment as first-line therapy for B-cell chronic lymphocytic leukemia. Leukemia 2004; 18: 484–90
Cao TM, Nanyen DD, Dugan K, et al. Incidence of cytomegalovirus (CMV) viremia during Campath-1H therapy for relapsed/refractory chronic lymphocytic leukemia (CLL) and prolymphocytic leukemia (PLL) [abstract 1540]. Blood 2001; 98 Suppl. 1: 366a
Lenihan DJ, Alencar AJ, Yang D, et al. Cardiac toxicity of alemtuzumab in patients with mycosis fungoides/Cezary Syndrome. Blood 2004; 104: 655–8
Rai K, Hallek M. Future prospects for alemtuzumab (MabCampath). Med Oncol 2002; (19 Suppl.): 57-63
Rai KR, Byrd JC, Peterson B, et al. Subcutaneous alemtuzumab following fludarabine for previously untreated patients with chronic lymphocytic leukemia (CLL) CALGB study 19901 [abstract 2506]. Blood 2003; 102 Suppl. 1: 672a
Tedeschi A, Montillo M, Cairoli R, et al. Successful CD34+ priming with intermediate-dose Ara-C (ID Ara-C) in chronic lymphytic leukemia (CLL) patients treated with sequential fludarabine (FAMP) and Campath-1H [abstract 3277]. Blood 2002; 100 Suppl. 1: 830a
Acknowledgments
This work was supported in part by a grant from the Medical University of Lodz (No. 503-106-2), and by the Foundation for the Development of Diagnostics and Therapy, Warsaw, Poland. The author has no conflicts of interest that are directly relevant to the content of this review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Robak, T. Alemtuzumab in the Treatment of Chronic Lymphocytic Leukemia. BioDrugs 19, 9–22 (2005). https://doi.org/10.2165/00063030-200519010-00002
Published:
Issue Date:
DOI: https://doi.org/10.2165/00063030-200519010-00002