Abstract
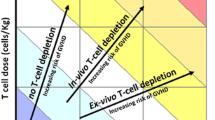
Graft versus host disease (GVHD) remains the main barrier to successful allogeneic bone marrow transplant outcomes. Depletion of graft T cells is an effective way of reducing the incidence of acute and chronic GVHD, and a variety of methods have been used to achieve this depletion. Donor CD8+ T cells seem to be the critical effector cells; GVHD is reduced when the depletion process eliminates these cells, but not when CD4 cells are targeted alone. However, despite the successful reduction in GVHD, transplant outcomes are usually inferior with T-cell depleted transplants, because of increased graft failure, infections and relapse. Alternative approaches are needed. In vivo T-cell depletion, using antithymocyte globulin (ATG) as part of the conditioning regimen, seems an attractive option. Pre-transplant ATG lingers in the bone marrow to deplete engrafting donor T cells, but also depletes host T cells to prevent graft rejection and allow de-escalation of the conditioning regimen. It also avoids the need for graft manipulation with its associated costs, need for expertise and CD34+ cell loss. The efficacy of pre-transplant horse ATG remains anecdotal but it has been reported to modestly lower GVHD in single arm studies. Rabbit ATG has been studied in prospective randomised trials. There is evidence of a dose-response effect in reducing GVHD; however, there was no improvement in outcome, because of increased mortality associated with infection. In contrast, pre-transplant alemtuzumab (campath-1H) or an earlier version of this molecule (campath-1G), which target CD52+ cells, do appear to be effective in reducing both acute and chronic GVHD. There is speculation that this is not solely due to the effect of campath on T cells but that it may also be due to the elimination of host antigen-presenting cells (APC), which seem to be important in GVHD pathogenesis. Host APC are more efficient at expressing endogenous and exogenous host antigens on class I MHC to donor CD8+ cells than donor APC, which need to cross-prime exogenous antigen. Campath-1G eliminates host dendritic cells by the time of graft infusion, supporting this as a possible mechanism of action. Pre-transplant alemtuzumab has not yet been studied in a prospective randomised study, and this is required to quantify any benefit on outcome; despite this, published studies do show cause for optimism.


Similar content being viewed by others
Notes
Use of tradenames is for product identification only and does not imply endorsement.
References
Ferrara JL, Deeg HJ. Graft-versus-host disease. N Engl J Med 1991; 324(10): 667–74
Gratwohl A, Hermans J, Apperley J, et al. Acute graft-versus-host disease: grade and outcome in patients with chronic myelogenous leukemia. Working Party Chronic Leukemia of the European Group for Blood and Marrow Transplantation. Blood 1995; 86(2): 813–8
Champlin RE, Passweg JR, Zhang MJ, et al. T-cell depletion of bone marrow transplants for leukemia from donors other than HLA-identical siblings: advantage of T-cell antibodies with narrow specificities. Blood 2000; 95(12): 3996–4003
Champlin R, Ho W, Gajewski J, et al. Selective depletion of CD8+ T lymphocytes for prevention of graft-versus-host disease after allogeneic bone marrow transplantation. Blood 1990; 76(2): 418–23
Nagler A, Condiotti R, Nabet C, et al. Selective CD4+ T-cell depletion does not prevent graft-versus-host disease. Transplantation 1998; 66(1): 138–41
Shlomchik WD, Couzens MS, Tang CB, et al. Prevention of graft versus host disease by inactivation of host antigen-presenting cells. Science 1999; 285(5426): 412–5
Roux E, Dumont-Girard F, Starobinski M, et al. Recovery of immune reactivity after T-cell-depleted bone marrow transplantation depends on thymic activity. Blood 2000; 96(6): 2299–303
Dumont-Girard F, Roux E, van Lier RA, et al. Reconstitution of the T-cell compartment after bone marrow transplantation: restoration of the repertoire by thymic emigrants. Blood 1998; 92(11): 4464–71
Marmont AM, Horowitz MM, Gale RP, et al. T-cell depletion of HLA-identical transplants in leukemia. Blood 1991 Oct 15; 78(8): 2120–30
Martin PJ, Hansen JA, Torok-Storb B, et al. Graft failure in patients receiving T cell-depleted HLA-identical allogeneic marrow transplants. Bone Marrow Transplant 1988; 3(5): 445–56
Ash RC, Horowitz MM, Gale RP, et al. Bone marrow transplantation from related donors other than HLA-identical siblings: effect of T cell depletion. Bone Marrow Transplant 1991; 7(6): 443–52
Aversa F, Tabilio A, Terenzi A, et al. Successful engraftment of T-cell-depleted haploidentical “three-loci” incompatible transplants in leukemia patients by addition of recombinant human granulocyte colony-stimulating factor-mobilized peripheral blood progenitor cells to bone marrow inoculum. Blood 1994; 84(11): 3948–55
Drobyski WR, Hessner MJ, Klein JP, et al. T-cell depletion plus salvage immunotherapy with donor leukocyte infusions as a strategy to treat chronic-phase chronic myelogenous leukemia patients undergoing HLA-identical sibling marrow transplantation. Blood 1999; 94(2): 434–41
Chakraverty R, Peggs K, Chopra R, et al. Limiting transplantation-related mortality following unrelated donor stem cell transplantation by using a nonmyeloablative conditioning regimen. Blood 2002; 99(3): 1071–8
Weiden PL, Doney K, Storb R, et al. Antihuman thymocyte globulin for prophylaxis of graft-versus-host disease: a randomized trial in patients with leukemia treated with HLA-identical sibling marrow grafts. Transplantation 1979; 27(4): 227–30
Doney KC, Weiden PL, Storb R, et al. Failure of early administration of antithymocyte globulin to lessen graft-versus-host disease in human allogeneic marrow transplant recipients. Transplantation 1981; 31(2): 141–3
Storb R, Blume KG, O’Donnell MR, et al. Cyclophosphamide and antithymocyte globulin to condition patients with aplastic anemia for allogeneic marrow transplantations: the experience in four centers. Biol Blood Marrow Transplant 2001; 7(1): 39–44
Bonnefoy-Berard N, Vincent C, Revillard JP. Antibodies against functional leukocyte surface molecules in polyclonal antilymphocyte and antithymocyte globulins. Transplantation 1991; 51(3): 669–73
Brennan DC, Flavin K, Lowell JA, et al. A randomized, double-blinded comparison of Thymoglobulin versus Atgam for induction immunosuppressive therapy in adult renal transplant recipients. Transplantation 1999; 67(7): 1011–8
Baurmann H, Revillard JP, Bonnefoy-Berard N, et al. Potent effects of ATG used as part of the conditioning in matched unrelated donor transplantation [abstract]. Blood 1998; 92(10 Suppl. 1): 1188a
Bacigalupo A, Lamparelli T, Bruzzi P, et al. Antithymocyte globulin for graft-versus-host disease prophylaxis in transplants from unrelated donors: 2 randomized studies from Gruppo Italiano Trapianti Midollo Osseo (GITMO). Blood 2001; 98(10): 2942–7
Perez-Simon JA, Kottaridis PD, Martino R, et al. Nonmyeloablative transplantation with or without alemtuzumab: comparison between 2 prospective studies in patients with lymphoproliferative disorders. Blood 2002; 100(9): 3121–7
Finke J, Bertz H, Schmoor C, et al. Allogeneic bone marrow transplantation from unrelated donors using in vivo anti-T-cell globulin. Br J Haematol 2000; 111(1): 303–13
Storb R, Leisenring W, Anasetti C, et al. Long-term follow-up of allogeneic marrow transplants in patients with aplastic anemia conditioned by cyclophosphamide combined with antithymocyte globulin. Blood 1997; 89(10): 3890–1
Storb R, Etzioni R, Anasetti C, et al. Cyclophosphamide combined with antithymocyte globulin in preparation for allogeneic marrow transplants in patients with aplastic anemia. Blood 1994; 84(3): 941–9
Riechmann L, Clark M, Waldmann H, et al. Reshaping human antibodies for therapy. Nature 1988; 332(6162): 323–7
Gilleece MH, Dexter TM. Effect of Campath-1H antibody on human hematopoietic progenitors in vitro. Blood 1993; 82(3): 807–12
Klangsinsirikul P, Carter GI, Byrne JL, et al. Campath-1G causes rapid depletion of circulating host dendritic cells (DCs) before allogeneic transplantation but does not delay donor DC reconstitution. Blood 2002; 99(7): 2586–91
Kirchhoff C, Schroter S. New insights into the origin, structure and role of CD52: a major component of the mammalian sperm glycocalyx. Cells Tissues Organs 2001; 168(1-2): 93–104
Harjunpaa A, Junnikkala S, Meri S. Rituximab (anti-CD20) therapy of B-cell lymphomas: direct complement killing is superior to cellular effector mechanisms. Scand J Immunol 2000; 51(6): 634–41
Shan D, Ledbetter JA, Press OW. Apoptosis of malignant human B cells by ligation of CD20 with monoclonal antibodies. Blood 1998; 91(5): 1644–52
Hale G, Hoang T, Prospero T, et al. Removal of T cells from bone marrow for transplantation: comparison of rat monoclonal anti-lymphocyte antibodies of different isotypes. Mol Biol Med 1983; 1(3): 305–19
Dyer MJ, Hale G, Hayhoe FG, et al. Effects of CAMPATH-1 antibodies in vivo in patients with lymphoid malignancies: influence of antibody isotype. Blood 1989; 73(6): 1431–9
Rowan W, Tite J, Topley P, et al. Cross-linking of the CAMPATH-1 antigen (CD52) mediates growth inhibition in human B- and T-lymphoma cell lines, and subsequent emergence of CD52-deficient cells. Immunology 1998; 95(3): 427–36
Albert ML, Pearce SF, Francisco LM, et al. Immature dendritic cells phagocytose apoptotic cells via alphavbeta5 and CD36, and cross-present antigens to cytotoxic T lymphocytes. J Exp Med 1998; 188(7): 1359–68
Kolb HJ, Schattenberg A, Goldman J, et al. Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients. Blood 1995 Sep 1; 86(5): 2041–50
Wing MG, Waldmann H, Isaacs J, et al. Ex-vivo whole blood cultures for predicting cytokine-release syndrome: dependence on target antigen and antibody isotype. Ther Immunol 1995; 2(4): 183–90
Wing MG, Moreau T, Greenwood J, et al. Mechanism of first-dose cytokine-release syndrome by CAMPATH 1-H: involvement of CD16 (FcgammaRIII) and CDlla/CD18 (LFA-1) on NK cells. J Clin Invest 1996; 98(12): 2819–26
Hale G, Waldmann H. Risks of developing Epstein-Barr virus-related lymphoproliferative disorders after T-cell-depleted marrow transplants. CAMPATH Users. Blood 1998; 91(8): 3079–83
Hale G, Zhang MJ, Bunjes D, et al. Improving the outcome of bone marrow transplantation by using CD52 monoclonal antibodies to prevent graft-versus-host disease and graft rejection. Blood 1998; 92(12): 4581–90
Kottaridis PD, Milligan DW, Chopra R, et al. In vivo CAMPATH-1H prevents graft-versus-host disease following nonmyeloablative stem cell transplantation. Blood 2000; 96(7): 2419–25
Giralt S, Thall PF, Khouri I, et al. Melphalan and purine analog-containing preparative regimens: reduced-intensity conditioning for patients with hematologic malignancies undergoing allogeneic progenitor cell transplantation. Blood 2001; 97(3): 631–7
Acknowledgements
No sources of funding were used to assist in the preparation of this review. The author has no conflicts of interest that are directly relevant to the content of this review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Simpson, D.R. T-Cell Depleting Antibodies. BioDrugs 17, 147–154 (2003). https://doi.org/10.2165/00063030-200317030-00001
Published:
Issue Date:
DOI: https://doi.org/10.2165/00063030-200317030-00001