Abstract
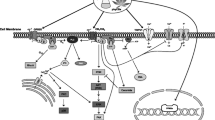
This review aims to improve understanding of the modulatory effects that cannabinoids exert on the immune system and CNS. Cannabinoids possess immunomodulatory activity, are neuroprotective in vivo and in vitro and can modify the production of inflammatory mediators, such as nitric oxide, prostanoids and cytokines, that are expressed by, and act on, the immune system and the brain. The mechanisms of cannabinoid actions are not fully understood, but appear to involve complex interactions between cannabinoid receptors and a number of signal transduction pathways. Endogenous cannabinoid ligands appear to act as local modulators of immune/inflammatory reactions.
Cannabinoid-induced immunosuppression may have implications for the treatment of neurological disorders that are associated with excess immunological activity, such as multiple sclerosis and Alzheimer’s disease. There is anecdotal evidence that cannabis use improves the symptoms of multiple sclerosis, and studies with animal models are beginning to provide evidence for the mechanism of such effects. The development of nonpsychotropic cannabinoid analogues and modulators of the metabolism of endogenous cannabinoid ligands may lead to novel approaches to the treatment of neurodegenerative disorders.
Similar content being viewed by others
References
Mechoulam R. The pharmacology of Cannabis sativa. In: Mechoulam R, editor. Cannabinoids as therapeutic agents. Boca Raton (FL): CRC, 1986: 1–19
Gaoni Y, Mechoulam R. Isolation, structure elucidation and partial synthesis of an active constituent of hashish. J Am Chem Soc 1964; 1646-47: 11–20
Matsuda LA, Lolait SJ, Brownstein MJ, et al. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 1990; 346: 561–4
Shire D, Carillon C, Kaghad M, et al. An amino-terminal variant of the central cannabinoid receptor resulting from alternative splicing. J Biol Chem 1995; 270: 3726–31
Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature 1993; 365: 61–5
Stefano GB, Liu Y, Goligorsky MS, et al. Cannabinoid receptors are coupled to nitric oxide release in invertebrate immunocytes, microglia, and human monocytes. J Biol Chem 1996; 271(32): 19238–42
Devane WA, Hanus L, Breuer A, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 1992; 258: 1946–9
Mechoulam R, Ben-Shabat S, Hanus L, et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol 1995; 50: 83–90
Felder CC, Veluz JS, Williams HL, et al. Cannabinoid agonists stimulate both receptor and non-receptor-mediated signal transduction pathways in cells transfected with and expressing cannabinoid receptor clones. Mol Pharmacol 1992; 42: 838–45
Kaminski NE, Abood ME, Kessler FK, et al. Identification of a functionally relevant cannabinoid receptor on mouse spleen cells that is involved in cannabinoid-mediated immune modulation. Mol Pharmacol 1992; 42: 736–42
Kiein TW, Friedman H, Specter S. Marijuana, immunity and infection. J Neuroimmunol 1998; 83: 102–15
Kiein TW, Newton C, Friedman H. Cannabinoid receptors and immunity. Imunol Today 1998; 19: 373–81
Jeon YJ, Yang KH, Pulaski JT, et al. Attenuation of inducible nitric oxide synthase gene expression by delta 9-tetrahydro-cannabinol is mediated through the inhibition of nuclear factor-kappa B/Rel activation. Mol Pharmacol 1996; 50: 334–41
Molina-Holgado F, Lledo A, Guaza C. Anandamide suppresses nitric oxide and TNF-alpha responses to Theiler’s virus or endotoxin in astrocytes. Neuroreport 1997; 8: 1929–33
Molina-Holgado F, Molina-Holgado E, Guaza C. The endogenous cannabinoid anandamide potentiates interleukin-6 production by astrocytes infected with Theiler’s murine encephalomyelitis virus by a receptor-mediated pathway. FEBS Lett 1998; 433: 139–42
Lyman WD, Sonett JR, Brosnan CF, et al. Delta 9-tetrahydro-cannabinol: a novel treatment for experimental autoimmune encephalomyelitis. J Neuroimmunol 1989; 23: 73–81
Wirguin I, Mechoulam R, Breuer A, et al. Suppression of experimental autoimmune encephalomyelitis by cannabinoids. Immunopharmacology 1994; 28: 209–14
Zheng ZM, Specter S. Suppression by delta-9-tetrahydrocannabinol of lipopolysaccharide induced and intrinsic tyrosine phosphorylation and protein expression in mouse peritoneal macrophages. Biochem Pharmacol 1994; 47: 2243–52
Juel-Jensen BE. Cannabis and recurrent herpes simplex [letter]. Br Med J 1972; 4(835): 296
Schatz AR, Koh WS, Kaminski NE. Delta 9-tetrahydrocannabinol selectively inhibits T-cell dependent humoral immune responses through direct inhibition of accessory T-cell function. Immunopharmacology 1993; 26: 129–37
Schatz AR, Lee M, Condie RB, et al. Cannabinoid receptors CB1 and CB2: a characterization of expression and adenylate cyclase modulation within the immune system. Toxicol Appl Pharmacol 1997; 142: 278–87
Lee M, Yang KH, Kaminski NE. Effects of putative cannabinoid receptor ligands, anandamide and 2-arachidonyl-glycerol, on immune function in B6C3F1 mouse splenocytes. J Pharmacol Exp Ther 1995; 275: 529–36
Lynn AB, Herkenham M. Localization of cannabinoid receptors and nonsaturable high-density cannabinoid binding sites in peripheral tissues of the rat: implications for receptor-mediated immune modulation by cannabinoids. J Pharmacol Exp Ther 1994; 268: 1612–23
Cabrai GA, Dove Pettit DA. Drugs and immunity: cannabinoids and their role in decreased resistance to infectious disease. J Neuroimmunol 1998; 83: 116–23
Cabrai GA, Lockmuller JC, Mishkin EM. Delta 9-tetrahydrocannabinol decreases alpha/beta interferon response to herpes simplex virus type 2 in the B6C3F1 mouse. Proc Soc Exp Biol Med 1986; 181(2): 305–11
Smith MS, Yamamoto Y, Newton C, et al. Psychoactive cannabinoids increase mortality and alter acute phase cytokine responses in mice sublethally infected with Legionella pneumophila. Proc Soc Exp Biol Med 1997; 214(1): 69–75
Ongradi J, Specter S, Horvath A, et al. Combined in vitro effect of marijuana and retrovirus on the activity of mouse natural killer cells. Pathol Oncol Res 1998; 4(3): 191–9
Coffey RG, Snella E, Johnson K, et al. Inhibition of macrophage nitric oxide production by tetrahydrocannabinol in vivo and in vitro. Int J Immunopharmacol 1996; 18(12): 749–52
Coffey RG, Yamamoto Y, Snella E, et al. Tetrahydrocannabinol inhibition of macrophage nitric oxide production. Biochem Pharmacol 1996; 52(5): 743–51
Ovadia H, Wohlman A, Mechoulam R, et al. Characterization of the hypothermic effect of the synthetic cannabinoid HU-210 in the rat. Relation to the adrenergic system and endogenous pyrogens. Neuropharmacology 1995; 34(2): 1175–80
Patrini G, Sacerdote P, Fuzio D, et al. Regulation of immune functions in rat splenocytes after acute and chronic in vivo treatment with CP-55,940, a synthetic cannabinoid compound. J Neuroimmunol 1997; 80(1-2): 1143–8
Nahas GG, Suciu-Foca N, Armand JP, et al. Inhibition of cellular mediated immunity in marihuana smokers. Science 1974; 183(123): 419–20
White SC, Brin SC, Janicki BW. Mitogen-induced blastogenic responses of lymphocytes from marihuana smokers. Science 1975; 188(4183): 71–2
Rachelefsky GS, Opelz G, Mickey MR, et al. Intact humoral and cell-mediated immunity in chronic marijuana smoking. J Allergy Clin Immunol 1976; 58(4): 483–90
Nahas GG, Oserman GS. Altered serum immunoglobulin concentration in chronic marijuana smokers. Adv Exp Biol Med 1991; 288: 25–32
Dax EM, Pilotte NS, Adler WH, et al. The effects of 9-ene-tetrahydrocannabinol on hormone release and immune function. J Steroid Biochem 1989; 34(1-6): 263–70
Massi P, Sacerdote P, Ponti W, et al. Immune function alterations in mice tolerant to delta9-tetrahydrocannabinol: functional and biochemical parameters. J Neuroimmunol 1998; 92(1-2): 60–6
Kawakami Y, Klein TW, Newton C, et al. Suppression by cannabinoids of a cloned cell line with natural killer cell activity. Proc Soc Exp Biol Med 1988; 187(3): 355–9
Ouyang Y, Hwang SG, Han SH, et al. Suppression of interleukin-2 by the putative endogenous cannabinoid 2-arachidonyl-glycerol is mediated through down-regulation of the nuclear factor of activated T cells. Mol Pharmacol 1998; 53: 676–83
Klein TW, Newton C, Friedman H. Cannabinoid receptors and the cytokine network. Adv Exp Biol Med 1998; 437: 215–22
Friedman H, Klein T, Specter S, et al. Drugs of abuse and virus susceptibility. Adv Biochem Psychopharmacol 1988; 44: 125–37
Burnette-Curley D, Cabrai GA. Differential inhibition of RAW264.7 macrophage tumoricidal activity by delta 9 tetra-hydrocannabinol. Proc Soc Exp Biol Med 1995; 210(1): 64–76
Gallily R, Yamin A, Waksman Y, et al. Protection against septic shock and suppression of tumor necrosis factor alpha and nitric oxide production by dexanabinol (HU-211), a non-psychotropic cannabinoid. J Pharmacol Exp Ther 1997; 283: 918–24
Varga K, Wagner JA, Bridgen DT, et al. Platelet and macro-phage-derived endogenous cannabinoids are involved in endotoxin-induced hypotension. FASEB J 1998; 11: 1035–44
Schwarz H, Blanco FJ, Lotz M. Anandamide, an endogenous cannabinoid receptor agonist inhibits lymphocyte proliferation and induces apoptosis. J Neuroimmunol 1994; 55:107–15
Zhu W, Friedman H, Klein TW. Delta9-tetrahydrocannabinol induces apoptosis in macrophages and lymphocytes: involvement of Bcl-2 and caspase-1. J Pharmacol Exp Ther 1998; 286(2): 1103–9
Zheng ZM, Specter S, Friedman H. Inhibition by delta-9-tetra-hydrocannabinol of tumor necrosis factor alpha production by mouse and human macrophages. Int J Immunopharmacol 1992; 14(8): 1445–52
Condie R, Herring A, Koh WS, et al. Cannabinoid inhibition of adenylate cyclase-mediated signal transduction and interleukin 2 (IL-2) expression in the murine T-cell line, EL4.IL-2. J Biol Chem 1996; 271: 13175–83
Herring AC, Koh WS, Kaminski NE. Inhibition of the cyclic AMP signaling cascade and nuclear factor binding to CRE and kappaB elements by cannabinol, a minimally CNS-active cannabinoid. Biochem Pharmacol 1998; 55(7): 1013–23
Snella E, Pross S, Friedman H. Relationship of aging and cytokines to the immunomodulation by delta-9-tetrahydro-cannabinol on murine lymphoid cells. Int J Immunopharmacol 1995; 17(112): 1045–54
Nakano Y, Pross S, Friedman H. Contrasting effect of delta-9-tetrahydrocannabinol on IL-2 activity in spleen and lymph node cells of mice of different ages. Life Sci 1993; 52: 41–51
Valk P, Verbakel S, Vankan Y, et al. Anandamide, a natural ligand for the peripheral cannabinoid receptor is a novel synergistic growth factor for hematopoietic cells. Blood 1997; 90: 1448–57
Berdyshev EV, Boichot E, Germain N, et al. Influence of fatty acid ethanolamides and delta9-tetrahydrocannabinol on cytokine and arachidonate release by mononuclear cells. Eur J Pharmacol 1997; 330(2–3): 231–40
Stefano GB, Salzet M, Rialas CM. Macrophage behavior associated with acute and chronic exposure to HIV GP 120, morphine and anandamide: endothelial implications. Int J Cardiol 1998; 64Suppl. 1: S3–13
Shivers SC, Newton C, Friedman H, et al. Delta 9-tetrahydro-cannabinol (THC) modulates IL-1 bioactivity in human monocyte/macrophage cell lines. Life Sci 1994; 54(17): 1281–9
Bouaboula M, Poinot-Chazel C, Bourrie B, et al. Activation of mitogen-activated protein kinases by stimulation of the central cannabinoid receptor CB1. Biochem J 1995; 312 (Pt 2): 637–41
Trisler K, Specter S. Delta-9-tetrahydrocannabinol treatment results in a suppression of interleukin-2-induced cellular activities in human and murine lymphocytes. Int J Immunopharmacol 1994; 16(7): 593–603
Kusher DI, Dawson LO, Taylor AC, et al. Effect of the psychoactive metabolite of marijuana, delta 9-tetrahydrocan-nabinol (THC), on the synthesis of tumor necrosis factor by human large granular lymphocytes. Cell Immunol 1994; 154(1): 99–108
Daaka Y, Zhu W, Friedman H, et al. Induction of interleukin-2 receptor alpha gene by delta9-tetrahydrocannabinol is mediated by nuclear factor kappaB and CB1 cannabinoid receptor. DNA Cell Biol 1997; 16(3): 301–9
Srivastava MD, Srivastava BI, Brouhard B. Delta9 tetrahydro-cannabinol and cannabidiol alter cytokine production by human immune cells. Immunopharmacology 1998; 40(3): 179–85
Bouaboula M, Poinot-Chazel C, Marchand J, et al. Signaling pathway associated with stimulation of CB2 peripheral cannabinoid receptor. Involvement of both mitogen-activated protein kinase and induction of Krox-24 expression. Eur J Biochem 1996; 237: 704–11
Wartmann M, Campbell D, Subramanian A, et al. The MAP kinase signal transduction pathway is activated by the endogenous cannabinoid anandamide. FEBS Lett 1995; 359: 133–6
Watzl B, Scuderi P, Watson RR. Marijuana components stimulate human peripheral blood mononuclear cell secretion of interferon-gamma and suppress interleukin-1 alpha in vitro. Int J Immunopharmacol 1991; 13(8): 1091–7
Bisogno T, Maurellis S, Melck D, et al. Biosynthesis, uptake, and degradation of anandamide and palmitoylethanolamide in leukocytes. J Biol Chem 1997; 272: 3315–23
Derocq JM, Segui M, Marchand J, et al. Cannabinoids enhance human B-cell growth at low nanomolar concentrations. FEBS Lett 1995; 369: 177–82
Fischer-Stenger K, Updegrove AW, Cabrai GA. Delta 9-tetrahydrocannabinol decreases cytotoxic T lymphocyte activity to herpes simplex virus type 1-infected cells. Proc Soc Exp Biol Med 1992; 200: 422–30
Fischer-Stenger K, Dove Pettit DA, et al. Delta 9-tetrahydro-cannabinol inhibition of tumor necrosis factor-alpha: suppression of post-translational events. J Pharmacol Exp Ther 1993; 267(3): 1558–65
Bouaboula M, Rinaldi M, Carayon P, et al. Cannabinoid-receptor expression in human leukocytes. Eur J Biochem 1993; 214(1): 173–80
Valk PJ, Delwel R. The peripheral cannabinoid receptor, Cb2, in retrovirally-induced leukemic transformation and normal hematopoiesis. Leuk Lymphoma 1998; 32(1–2): 29–43
Felder CC, Glass M. Cannabinoid receptors and their endogenous agonists. Annu Rev Pharmacol Toxicol 1998; 38: 179–200
Gonzalez GA, Montminy MR. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell 1989; 59: 675–80
Derocq JM, Bouaboula M, Marchand J, et al. The endogenous cannabinoid anandamide is a lipid messenger activating cell growth via a cannabinoid receptor-independent pathway in hematopoietic cell lines. FEBS Lett 1998; 425: 419–25
Rinaldi-Carmona M, Barth F, Millan J, et al. SR 144528, the first potent and selective antagonist of the CB2 cannabinoid receptor. J Pharmacol Exp Ther 1998; 284: 644–50
Stuehr DJ, Nathan CF. Nitric oxide. A macrophage product responsible for cytostasis and respiratory inhibition in tumor target cells. J Exp Med 1989; 169: 1543–55
Feng L, Sun W, Xia Y, et al. Cloning two isoforms of rat cyclooxygenase: differential regulation of their expression. Arch Biochem Biophys 1993; 307(2): 361–8
Yu M, Ives D, Ramesha CS. Synthesis of prostaglandin E2 ethanolamide from anandamide by cyclooxygenase-2. J Biol Chem 1997; 272: 21181–6
Shen M, Thayer SA. Cannabinoid receptor agonists protect cultured rat hippocampal neurons from excitotoxicity. Mol Pharmacol 1998; 54(3): 459–62
Nagayama T, Sinor AD, Simon RP, et al. Cannabinoids and neuroprotection in global and focal cerebral ischemia and in neuronal cultures. J Neurosci 1999; 19(8): 2987–95
Blazquez C, Sanchez C, Daza A, et al. The stimulation of ketogenesis by cannabinoids in cultured astrocytes defines carnitine palmitoyltransferase I as a new ceramide-activated enzyme. J Neurochem 1999; 72(4): 1759–68
Sanchez C, Galve-Roperh I, Rueda D, et al. Involvement of sphingomyelin hydrolysis and the mitogen-activated protein kinase cascade in the delta-9-tetrahydrocannabinol-induced stimulation of glucose metabolism in primary astrocytes. Mol Pharmacol 1998; 54(5): 834–43
Molina-Holgado F, Grencis R, Rothwell NJ. Interactions of cannabinoids and anti-inflammatory cytokines in astrocyte mice cultures. Soc Neurosci Abstr 1999; 25(1): 1180
Sanchez C, Galve-Roperh I, Canova C, et al. Delta-9-tetrahy-drocannabinol induces apoptosis in C6 glioma cells. FEBS Lett 1998; 436(1): 6–10
Waksman Y, Olson JM, Carlisle SJ, et al. The central cannabinoid receptor (CB1) mediates inhibition of nitric oxide production by rat microglial cells. J Pharmacol Exp Ther 1999; 288(3): 1357–66
Shohami E, Gallily R, Mechoulam R, et al. Cytokine production in the brain following closed head injury: dexanabinol (HU-211) is a novel TNF-alpha inhibitor and an effective neuroprotectant. J Neuroimmunol 1997; 72(2): 169–77
Hunter SA, Audette CA, Burstein S. Elevation of brain prostaglandin E2 levels in rodents by delta 1-tetrahydrocannabinol. Prostaglandins Leukot Essent Fatty Acids 1991; 43(3): 185–90
Di Marzo V, Fontana A, Cadas H, et al. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature 1994; 372(6507): 686–91
Deutsch DG, Chin SA. Enzymatic synthesis and degradation of anandamide, a cannabinoid receptor agonist. Biochem Pharmacol 1993; 46(5): 791–6
De Petrocelli L, Melck D, Ueda N, et al. Novel inhibitors of brain, neuronal, and basophilic anandamide amidohydrolase. Biochem Biophys Res Commun 1997; 231: 82–88
Bisogno T, Berrendero F, Ambrosino G, et al. Brain regional distribution of endocannabinoids: implications for their biosynthesis and biological function. Biochem Biophys Res Commun 1999; 256: 377–80
Merrill JE, Chen IS. HIV-1, macrophages, glial cells, and cytokines in AIDS nervous system disease. FASEB J 1991; 5(10): 2391–7
Patterson PH. Cytokines in Alzheimer’s disease and multiple sclerosis. Curr Opin Neurobiol 1995; 5(5): 642–6
Hayashi Y, Nomura M, Yamagishi S, et al. Induction of various blood-brain barrier properties in non-neural endothelial cells by close apposition to co-cultured astrocytes. Glia 1997; 19(1): 13–26
Fabry Z, Raine CS, Hart MN. Nervous tissue as an immune compartment: the dialect of the immune response in the CNS. Immunol Today 1994; 15(5): 218–24
Merrill JE, Benveniste EN. Cytokines in inflammatory brain lesions: helpful and harmful. Trends Neurosci 1996; 19(8): 331–8
Bouaboula M, Bourrie B, Rinaldi-Carmona M, et al. Stimulation of cannabinoid receptor CB1 induces krox-24 expression in human astrocytoma cells. J Biol Chem 1995; 270(23): 13973–80
Rinaldi-Carmona M, Barth F, Heaulme M, et al. SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett 1994; 350(2–3): 240–4
Rothwell NJ, Hopkins SJ. Cytokines and the nervous system II: actions and mechanisms of action. Trends Neurosci 1995; 18(3): 130–6
Rothwell N, Allan S, Toulmond S. The role of interleukin-1 in acute neurodegeneration and stroke: pathophysiological and therapeutic implications. J Clin Invest 1997; 100(11): 2648–52
Aschner M. Immune and inflammatory responses in the CNS: modulation by astrocytes. Toxicol Lett 1998; 102–103: 283–7
Raine CS, Bonetti B, Cannella B. Multiple sclerosis: expression of molecules of the tumor necrosis factor ligand and receptor families in relationship to the demyelinated plaque. Rev Neurol (Paris) 1998; 154(8–9): 577–85
Selmaj K, Walczak A, Mycko M, et al. Suppression of experimental autoimmune encephalomyelitis with a TNF binding protein (TNFbp) correlates with downregulation of VCAM-1/VLA-4. Eur J Immunol 1998; 28(6): 2035–44
Hofman FM, Hinton DR, Johnson K, et al. Tumor necrosis factor identified in multiple sclerosis brain. J Exp Med 1989; 170(2): 607–12
Navikas V, Matusevicius D, Soderstrom M. Increased interleukin-6 mRNA expression in blood and cerebrospinal fluid mononuclear cells in multiple sclerosis. J Neuroimmunol 1996; 64(1): 63–9
Tilg H, Dinarello CA, Mier JW. IL-6 and APPs: anti-inflammatory and immunosuppressive mediators. Immunol Today 1997; 18(9): 428–32
Benveniste EN, Tang LP, Law RM, et al. Differential regulation of astrocyte TNF-alpha expression by the cytokines TGF-beta, IL-6 and IL-10. Int JDev Neurosci 1995; 13(3–4): 341–9
Consroe P, Musty R, Rein J, et al. The perceived effects of smoked cannabis on patients with multiple sclerosis. Eur Neurol 1997; 38(1): 44–8
Leker RR, Shohami E, Abramsky O, et al. Dexanabinol: a novel neuroprotective drug in experimental focal cerebral ischemia. J Neurol Sci 1999; 162(2): 114–9
Biegon A. Neuroprotective activity of HU-211, a novel non-psychotropic synthetic cannabinoid. Ann NY Acad Sci 1995; 765: 314
Westlake TM, Howlett AC, Bonner TI, et al. Cannabinoid receptor binding and messenger RNA expression in human brain: an in vitro receptor autoradiography and in situ hybridization histochemistry study of normal aged and Alzheimer’s brains. Neuroscience 1994; 63: 637–52
Volicer L, Stelly M, Morris J, et al. Effects of dronabinol on anorexia and disturbed behavior in patients with Alzheimer’s disease. Int J Geriatr Psychiatry 1997; 12: 913–9
Berglund BA, Boring DL, Wilken GH, et al. Structural requirements for arachidonylethanolamide interaction with CB 1 and CB2 cannabinoid receptors: pharmacology of the carbonyl and ethanolamide groups. Prostaglandins Leukot Essent Fatty Acids 1998; 59: 111–8
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Molina-Holgado, E., Guaza, C., Borrell, J. et al. Effects of Cannabinoids on the Immune System and Central Nervous System. BioDrugs 12, 317–326 (1999). https://doi.org/10.2165/00063030-199912050-00001
Published:
Issue Date:
DOI: https://doi.org/10.2165/00063030-199912050-00001