Abstract
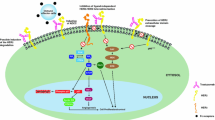
▴ Trastuzumab is a recombinant humanised monoclonal antibody specific for the growth factor receptor p185HER2 (HER2) which is overexpressed in 25 to 30% of breast cancer tumours.
▴ The drug inhibits the growth of human breast cancer cells overexpressing HER2 in vitro and in vivo. It shows additive antitumour activity in vitro and in vivo when administered with paclitaxel, doxorubicin, various cytokines or tamoxifen.
▴ In patients with metastatic breast cancer whose tumours overexpressed HER2, trastuzumab (4 mg/kg loading dose then 2 mg/kg/week by intravenous infusion) produced objective responses in 21% of 213 patients. A further 7% of patients had minor responses and 30% had stable disease.
▴ Combination therapy with trastuzumab and either paclitaxel or doxorubicin (or epirubicin) plus cyclophosphamide produced a higher response rate (49%), longer median time to disease progression (7.6 months), a higher one-year survival rate (78%) and significantly increased median overall survival (25.4 months) than antineoplastic agents alone (response rate 32%, time to disease progression 4.6 months, one-year survival rate 67% and overall survival 20.3 months) in a phase III study in 469 patients.
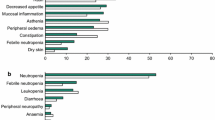
▴ Trastuzumab is generally well tolerated. Chills, fever, nausea, vomiting, weakness and headache were among the most common adverse events in clinical trials and occurred in 40 to 50% of patients during the first infusion of the drug. Cardiac dysfunction was the most serious adverse event reported and was more common in patients receiving trastuzumab plus antineoplastic therapy than in those receiving trastuzumab alone.
Similar content being viewed by others
References
Pegram MD, Lipton A, Hayes DF, et al. Phase II study of receptor-enhanced chemosensitivity using recombinant humanized anti-p185HER2/ neu monoclonal antibody plus cisplatin in patients with HER2/neu-overexpressing metastatic breast cancer refractory to chemotherapy treatment. J Clin Oncol 1998 Aug; 16: 2659–71
Baselga J, Tripathy D, Mendelsohn J, et al. Phase II study of weekly intravenous recombinant humanized anti-p185HER2 neu monoclonal antibody in patients with HER2/neu-overexpressing metastatic breast cancer. J Clin Oncol 1996 Mar; 14: 737–44
Slamon DJ, Godolphin W, Jones LA, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 1989; 244: 707–12
Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: Correlation of relapse and survival with amplification of the HER2/neu. oncogene. Science 1987; 235: 177–82
Press MF, Bernstein L, Thomas PA, et al. HER-2/neu gene amplification characterized by fluorescence in situ hybridization: poor prognosis in node-negative breast carcinomas. J Clin Oncol 1997; 15: 2894–904
Seshadri R, Firgaira FA, Horsfall DJ, et al. Clinical significance of HER-2/neu oncogene amplification in primary breast cancer. The South Australian Breast Cancer Study Group. J Clin Oncol 1993; 11: 1936–42
Genentech I. FDA advisory committee recommends approval of first monoclonal antibody for metastatic breast cancer. In: Media Release 1998 Sep: [3pages],2
Baselga J, Norton L, Albanell J, et al. Recombinant humanized anti-HER2 antibody (Herceptin™ enhances the antitumor activity of paclitaxel and doxorubicin against HER2/neu overexpressing human breast cancer xenografts. Cancer Res 1998 Jul 1; 58: 2825–31
Carter P, Presta L, Gorman CM, et al. Humanization of an anti-p185HER2 antibody for human cancer therapy. Proc Natl Acad Sci U S A 1992; 89: 4285–9
Pegram M, Hsu S, Lewis G, et al. Inhibitory effects of combinations of HER-2/neu antibody and chemotherapeutic agents used for treatment of human breast cancers. Oncogene 1999; 18: 2241–51
Kopreski MS, Lipton A, Harvey HA, et al. Growth inhibition of breast cancer cell lines by combinations of anti-P185HER2 monoclonal antibody and cytokines. Anticancer Res 1996 Jan–Feb; 16: 433–6
Witters LM, Kumar R, Chinchilli VM, et al. Enhanced anti-proliferative activity of the combination of tamoxifen plus HER-2-neu antibody. Breast Cancer Res Treat 1997 Jan; 42: 1–5
Lopez AM, Pegram MD, Landaw EM, et al. Models to assess combination therapy interactions: optimal timing of anti-HER-2 antibody and doxorubicin in breast cancer [abstract]. 88th Annu Meet Am Assoc Cancer Res 1997 Apr 12: 605
Park JW, Colbern G, Nuijens A, et al. Increased levels of circulating HER2 ECD in response to anti-HER2 antibody therapy [abstract]. Breast Cancer Res Treat 1997 Oct; 46: 67
Cobleigh MA, Vogel CL, Tripathy D, et al. Efficacy and safety of Herceptin™ (humanized anti-HER2 antibody) as a single agent in 222 women with HER2 overexpression who relapsed following chemotherapy for metastatic breast cancer [abstract]. 34th Proc Am Soc Clin Oncol 1998 May 16; 17: 97
Genentech Inc. Herceptin® (trastuzumab) prescribing information. San Francisco (CA), 1998
Slamon D, Leyland-Jones B, Shak S, et al. Addition of Herceptin™ (humanized anti-HER2 antibody) to first line chemotherapy for HER2 overexpressing metastatic breast cancer markedly increases anticancer activity: a randomized, multinational controlled phase III trial [abstract]. 34th Proc Am Soc Clin Oncol 1998 May 16; 17: 98
Baselga J, for the Herceptin International Investigator Group. Multinational studies of HerceptinTM (humanized anti-HER2 antibody) in HER+ metastatic breast cancer: Phase III of Herceptin plus chemotherapy (CRx) vs CRx alone in first-line and large phase II of Herceptin alone in advanced disease. Ann Oncol 9 (Suppl. 2)
Data on file. Hoffmann-La Roche, Basel. 1999
Norton L, Slamon D, Leyland Jones B, et al. Overall survival advantage to simultaneous chemotherapy plus the humanized anti-HER2 monoclonal antibody herceptin in HER2-overexpressing metastatic breast cancer [abstract]. 35th Am Soc Clin Oncol 1999; 18: 127
Sliwkowski MX, Fox JA, Twaddell T et al. A humanized monoclonal antibody for the treatment of HER2 overexpressing breast cancer [abstract]. 87th Annu Meet Am Assoc Cancer Res 1996 Apr 20: 625-6
FDA clears Herceptin at record speed. Scrip Mag 1998; 2374: 20
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Perry, C.M., Wiseman, L.R. Trastuzumab. BioDrugs 12, 129–135 (1999). https://doi.org/10.2165/00063030-199912020-00004
Published:
Issue Date:
DOI: https://doi.org/10.2165/00063030-199912020-00004