Summary
Neoral® is a novel formulation in which cyclosporin is suspended in a uniform, fine microemulsion by a combination of lipophilic solvent, hydrophilic solvent and surfactant. Prospective randomised double-blind clinical trials have confirmed that absorption of cyclosporin is more rapid, complete and consistent from Neoral®than from previous formulations (such as Sandimmun®). Use of Neoral® for acute or maintenance immunosuppression with conventional trough concentrations has shown no evidence of long term renal or other toxicity.
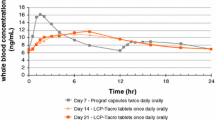
The prospective, randomised multicentre pharmaco-epidemiological study performed in 1097 stable Canadian renal transplant patients under conditions of normal clinical practice has shown that patients can be safely switched from using gelatin capsules to Neoral®, and has confirmed the pharmacokinetic advantages observed in de novo use. Peak cyclosporin concentration occurred at 1.6 vs 2.8 hours and was increased by 69% (1063 vs 627 µg/L), and exposure to cyclosporin was increased by 45% (5215 vs 3579 µg/L·h). Patients who were more than 1 standard deviation below the mean of absorption when they were receiving Sandimmun® had an almost 3-fold increase in absorption when taking Neoral®, and by 90 days postconversion all poor absorbers had become good or excellent absorbers on Neoral®. The variability of drug exposure in patients receiving cyclosporin is also significantly decreased in patients receiving Neoral® and there appears to be no long term adverse consequence of switching to Neoral®.
Preliminary pharmacokinetic data suggest that the pharmacokinetic advantages of Neoral® may also translate into reduced healthcare costs, but confirmation in formal analyses is required. The current information suggests that the more complete and consistent absorption of cyclosporin provided by Neoral® offers the opportunity for precise and effective long term immunosuppression, a critical advantage in view of the emerging relationship between variable drug exposure and long term immunological injury. However, trough concentration monitoring appears inadequate to fully exploit these advantages, and the use of a simple limited sampling strategy is emerging as an important tool for predicting drug exposure.
Similar content being viewed by others
Références
Mueller EA, Kovarik JM, Van Bree JB, et al. Pharmacokinetics and tolerability of a microemulsion formulation of cyclosporine in renal transplant recipients: a concentration controlled comparison with the commercial formulation. Transplantation 1994; 57: 1178
Keown PA. A randomized prospective double-blind multicenter study of cyclosporine microemulsion (Sandimmune Neoral) in de novo renal transplantation. Proceedings of the 14th Annual Meeting of the American Society of Transplant Physicians; 1995 May 14–17; Chicago: 74
Kovarik JM, Mueller EA, Richard F, et al. Evidence for earlier stabilization of cyclosporine pharmacokinetics in de novo transplant patients receiving a microemulsion formulation. Transplantation 1996; 62: 759–63
Barone G, Martin Bunke C, Choc MC, et al. The safety and tolerability of cyclosporine emulsion versus cyclosporine in a randomised, double-blind comparison in primary renal graft recipients. Transplantation 1996; 61: 968
The Canadian Neoral Study Group. A randomized prospective multicenter pharmacoepidemiologic study of Neoral (cyclosporine microemulsion) in stable renal graft recipients: results at 18 months [abstract no. 576]. Proceedings of the 16th Meeting of the American Society of Transplant Physicians; 1997 May 10–14; Chicago: 228
Keown P, Landsberg D, Halloran P, et al. A randomized, prospective multicentre pharmacoepidemiologic study of cyclosporine microemulsion in stable renal graft recipients. Transplantation 1996; 62: 1744–52
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Keown, P.A. Apport du Néoral® en transplantation rénale. BioDrugs 8 (Suppl 1), 12–14 (1997). https://doi.org/10.2165/00063030-199700081-00007
Published:
Issue Date:
DOI: https://doi.org/10.2165/00063030-199700081-00007