Abstract
Objective: Succinylated gelatin is widely used in the management of hypovolaemic conditions. Volplex® is a new presentation of succinylated gelatin in a flexible, collapsible bag, which is sterilised at 121°C. This study aimed to document the safety and tolerability of Volplex®, using Gelofusine® as a control, in elective surgery.
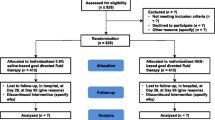
Design and patients: The study was a single-centre, open-label, randomised, parallel-group trial that included patients aged 18–75 years who were, in the opinion of the investigator, likely to need an infusion of a colloid as part of their surgical management. The patients were randomised to receive either Volplex® or Gelofusine® in a 2: 1 ratio in favour of Volplex®. Three assessments, preoperative, perioperative and 24 hours following anaesthetic induction, were performed. All statistical testing was two-sided and performed at a 5% level of significance.
Results: 76 patients (52 receiving Volplex® and 24 receiving Gelofusine®) were included in the intent-to-treat population. The volumes of fluids infused were 1154mL and 1115mL for the Volplex® and Gelofusine® groups, respectively, of which 962mL was Volplex® and 958mL was Gelofusine®, respectively. Patients also received crystalloids and blood and there were no significant differences between groups in the amounts received. Eighteen Volplex® recipients (35%) and 12 Gelofusine® recipients (50%) reported at least one adverse event. Of the 27 events reported for Volplex®, 15 (56%) were considered not related and 12 (44%) possibly related to Volplex®. In the case of Gelofusine®, 17 events were reported, and of these eight (47%) were considered not related and nine (53%) possibly related to Gelofusine®. None of the reported adverse events were considered probably related to either colloid. One serious adverse event, mild hypothermia, was reported in the Gelofusine® group. The unexpected length of surgery was stated as the causal factor. There were no significant differences between Volplex® and Gelofusine® with regard to the number of patients with out-of-range laboratory values postoperatively. Urine output for both groups was reduced postoperatively, but there were no major differences between the groups observed.
Conclusion: This small, open-label study showed that Volplex®, a new presentation of succinylated gelatin, was comparable to a well-established brand, Gelofusine® when used in the management of patients undergoing elective surgery.






Similar content being viewed by others
Notes
The use of tradenames is for product identification purposes only and does not imply endorsement.
References
Maclntyre E, Bullen C, Machin SJ. Fluid replacement in hypovolaemia. Intensive Care Med 1985; 11: 231–3
Bailliere T. Plasma volume support. In: Haljamae H, editor. Bailliere’s clinical anaesthesiology international practice and research. London: WB Saunders Company Ltd, 1997: 15–48
Dollery C. Gelatin plasma substitutes. In: Therapeutic drugs. Vol. 2. Edinburgh: Churchill Livingstone, 1991: G7–10
Lundsgaard-Hansen P, Tschirren B. Modified gelatin as a plas-ma substitute. In: Jamieson GA, Greenwalt TJ, editors, Blood substitutes and plasma expanders. New York: Alan R. Liss, 1978: 227–57
Edwards JD, Nightingale P, Wilkins RG, et al. Hemodynamic and oxygen transport response to modified gelatin in critically ill patients. Crit Care Med 1989; 17: 394–8
Lundsgaard-Hansen P, Tschirren B. Modified fluid gelatin as a plasma substitute. In: Jamieson GA, Greenwalt TJ, editors. Blood substitutes and plasma expanders. New York: Alan R. Liss 1978: 227–57
Hogan JJ. The intravenous use of colloidal (gelatin) solutions in shock. JAMA 1915; 64: 721–6
Richter W, Hedin H. Solutions and emulsions used for intravenous infusions. Handbook Exp Pharmacol 1983; 63: 581–628
Ramsay G. Intravenous volume replacement: indications and choices. BMJ 1988; 296: 1422–3
Rudowski WJ. Evaluation of modern plasma expanders and blood substitutes. Br J Hosp Med 1980; 23: 389–99
Yates DW. Volume replacement: the choice of fluid. Hosp Update 1987 Apr 13: 297–306
Mishler JM. Synthetic plasma volume expanders: their pharmacology, safety and clinical efficacy. Clin Haematol 1984; 13: 75–92
Physiological response to infection, trauma and surgery [online]. Available from http://www.surgical-tutor.org.uk/defaulthome.htm.core/preop2/physiology.htm~right [Accessed 2003 7 Dec]
Messmer KFW. The use of plasma substitutes with special attention to their side effects. World J Surg 1987; 11: 69–74
Acknowledgements
This study was carried out at the Department of Anaesthesia, Derriford Hospital, Plymouth, Devon, UK. The principal investigator was Prof. J. Robert Sneyd. Maelor Pharmaceuticals Ltd, Riversdale, Newbridge, Wrexham, UK funded the work. Maelor Pharmaceuticals Ltd would like to thank Professor Sneyd, all those who assisted in the completion of the study, the patients who took part in the study, and Bernard Dean for providing the first draft of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sneyd, R.J., Dean, B.C. & Dwyer, C. Safety and Tolerability of Volplex® in Elective Surgery. Clin. Drug Investig. 24, 73–79 (2004). https://doi.org/10.2165/00044011-200424020-00002
Published:
Issue Date:
DOI: https://doi.org/10.2165/00044011-200424020-00002