Abstract
This paper reports the clinical pharmacokinetics of artemether and lumefantrine in healthy volunteers and in malaria patients. These two drugs are the active components of the fixed-dose oral combination tablet co-artemether (Riamet®), used for the treatment of Plasmodium falciparum malaria.
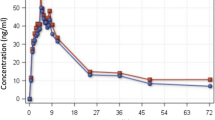
Artemether has a fast absorption rate followed by rapid clearance from plasma [terminal elimination half-life (t1/2β) 2 to 3 hours]. Its major metabolite dihydroartemisinin (DHA) is formed rapidly and has a similar clearance pattern to artemether. Lumefantrine is slowly absorbed (2 hours lag time) followed by a slow clearance from plasma (t1/2β up to 10 days). Food intake significantly increases the bioavailability of both artemether (>2-fold) and lumefantrine (approximately 16-fold).
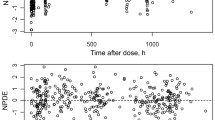
Artemether and lumefantrine are predominantly metabolised by the cytochrome P450 3A4 (CYP3A4) isoenzyme. The intersubject variability for artemether, DHA and lumefantrine is high in both healthy volunteers and patients.
CYP3A4 substrates/inhibitors such as the antimalarial mefloquine can be coadministered with co-artemether without clinically relevant risk of drug-drug interaction. Similarly, potential for a significant medical hazard in the management of P. falciparum malaria with co-artemether following co-administration in close temporal relationship with quinine (a CYP3A4 substrate) is unlikely. The wide therapeutic index of artemether and lumefantrine and the short duration of administration of co-artemether suggest that the risk for any tolerability problems from drug accumulation is minimal.
Similar content being viewed by others
References
Wernsdorfer WH. Epidemiology of drag resistance in malaria (1994–1995). Geneva, World Health Organisation; 1993 Sep 27–29. Report no.: WHO/MAL/94.1067
Olliaro PL, Trigg PI. Status of antimalarial drags under development. Bull World Health Organ 1995; 73(5): 565–71
Karbwang J, Na-Bangchang K. Clinical pharmacokinetics of halofantrine. Clin Pharmacokinet 1994 Aug; 27(2): 104–19
Toivonen L, Viitasalo M, Siikamaki H, et al. Provocation of ventricular tachycardia by antimalarial drug halofantrine in congenital long QT syndrome. Clin Cardiol 1994 Jul; 17(7): 403–4
Monlun E, Le Metayer P, Szwandt S, et al. Cardiac complications of halofantrine: a prospective study of 20 patients. Trans R Soc Trop Med Hyg 1995 Jul–Aug; 89(4): 430–3
Nosten F, ter Kuile FO, Luxemburger C, et al. Cardiac effects of antimalarial treatment with halofantrine. Lancet 1993 Apr 24; 341(8852): 1054–6
Bunnag D, Viravan C, Looareesuwan S, et al. Clinical trial of artesunate and artemether on multidrug resistant falciparum malaria in Thailand. A preliminary report. Southeast Asian J Trop Med Public Health 1991 Sep; 22(3): 380–5
Karbwang J, Na-Bangchang K, Thanavibul A, et al. Pharmacokinetics of mefloquine alone or in combination with artesunate. Bull World Health Organ 1994; 72(1): 83–7
White NJ. The treatment of malaria. N Engl J Med 1996 Sep 12; 335(11): 800–6
Ezzet F, Mull R, Karbwang J. Population pharmacokinetics and therapeutic response of CGP 56697 (artemether + benflumetol) in patients. Br J Clin Pharmacol 1998 Dec; 46(6): 553–561
Hien TT, White NJ. Qinghaosu. Lancet 1993 Mar 6; 341(8845): 603–8
von Seidlein L, Jaffar S, Pinder M, et al. Treatment of African children with uncomplicated falciparum malaria with a new antimalarial drug, CGP 56697. J Infect Dis 1997 Oct; 176(4): 1113–6
van Vugt M, Brockman A, Gemperli B, et al. Randomized comparison of artemether-benflumetol and artesunate-mefloquine in treatment of multidrug-resistant falciparum malaria. Antimicrob Agents Chemother 1998 Jan; 42(1): 135–9
Hatz C, Habdulla S, Mull R, et al. Efficacy and safety of CGP 56697 (artemether and benflumetol) compared with chloroquine to treat acute falciparum malaria in Tanzanian children aged 1–5 years. Trop Med Int Health 1998 Jun; 3(6): 498–504
Jiao x, Liu GY, Shan CO, et al. Phase II trial in China of a new, rapidly-acting and effective oral antimalarial, CGP 56697, for the treatment of Plasmodium falciparum malaria. Southeast Asian J Trop Med Public Health 1997 Sep; 28(3): 476–81
Looareesuwan S, Wilairatana P, Chokejindachai W, et al. A randomized, double-blind, comparative trial of a new oral combination of artemether and benflumetol (CGP 56697) with mefloquine in the treatment of acute Plasmodium falciparum malaria in Thailand. Am J Trop Med Hyg 1999 Feb; 60(2): 238–43
van Vugt M, Wilairatana P, Gemperli B, et al. Efficacy of six doses of artemether-lumefantrine (benflumetol) in multi-drug resistant falciparum malaria. Am J Trop Med Hyg 1999; 60(6): 936–42
Navaratnam V, Mansor SM, Chin LK, et al. Determination of artemether and dihydroartemisinin in blood plasma by HPLC for application in clinical pharmacological studies. J Chromatogr 1995 Jul 21; 669(2): 289–94
van Agtmael MA, Butter JJ, Portier EJ, et al. Validation of an improved reversed-phase high-performance liquid chromatography assay with reductive electrochemical detection for the determination of artemisinin derivatives in man. Ther Drug Monit 1998 Feb; 20(1): 109–16
Zeng MY, Lu ZL, Yang SC, et al. Determination of benflumetol in human plasma by reversed-phase high-performance liquid chromatography with ultraviolet detection. J Chromatogr 1996 Jun 7; 681(2): 299–306
Mansor SM, Navaratnam V, Yahaya N, et al. Determination of a new antimalarial drug, benflumetol, in blood plasma by high-performance liquid chromatography. J Chromatogr 1996 Jul 12; 682(2): 321–5
Na-Bangchang K, Karbwang J, Thomas CG, et al. Pharmacokinetics of artemether after oral administration to healthy Thai males and patients with acute uncomplicated falciparum malaria. Br J Clin Parmacol 1994 Mar; 37(3): 249–53
Mordi MN, Mansor SM, Navaratnam V, et al. Single dose pharmacokinetics of oral artemether in healthy Malaysian volunteers. Br J Clin Pharmacol 1997 Apr; 43(4): 363–5
Karbwang J, Na-Bangchang K, Thanavibul A, et al. Plasma concentrations of artemether and its major plasma metabolite, dihydroartemisinin, following a 5-day regimen of oral artemether, in patients with uncomplicated falciparum malaria. Ann Trop Med Parasitol 1998 Jan; 92(1): 31–6
Colussi D, Parisot C, Legay F, et al. Binding of artemether and lumefantrine to plasma proteins and erythrocytes. Eur J Pharma Sci 1999 Sept; 9(1): 9–16
Maulard C, Urien S, Bastian G, et al. Binding of retelliptine, a new antitumoral agent, to serum proteins and erythrocytes. Biochem Pharmacol 1990 Aug 15; 40(4): 895–8
Colussi D, Parisot C, Lefèvre G. Binding of iralukast to serum proteins and erythrocytes: measurements using ultrafiltration and an erythrocyte partitioning method. Eur J Pharm Sci 1998; 7(2): 167–73
Bindschedler M, Degen P, Lu ZL, et al. Comparative bioavailability of benflumetol after administration of single oral doses of co-artemether under fed and fasted conditions to healthy subjects [abstract no. P-01-96]. XIVth International Congress for Tropical Medicine and Malaria; 1996 Nov 17–22; Nagasaki, Japan, 346
Skelton-Stroud PN, Wernsdorfer WH, Ward SA, et al. The preclinical safety of Riamet® — a new combination of antimalarial [abstract no. C15]. 6th Conference of the International Society of Travel Medicine; 1999 Jun 6–10; Montreal, Quebec, Canada
van Agtmael MA, van der Graaf CAA, Dien TK, et al. The contribution of the enzymes CYP2D6 and CYP2C19 in the demethylation of artemether in healthy subjects. Eur J Drug Metab Pharmacokinet 1998 Jul–Sep; 23(3): 429–36
Teja-Isavadharm P, Nosten F, Kyle DE, et al. Comparative bioavailability of oral, rectal, and intramuscular artemether in healthy subjects: use of simultaneous measurement by high performance liquid chromatography and bioassay. Br J Clin Pharmacol 1996 Nov; 42(5): 599–604
White NJ, Olliaro P. Artemisinin and derivatives in the treatment of uncomplicated malaria. Med Trop (Mars) 1998; 58(3 Suppl.): 54–6
Karbwang J, Na-Bangchang K, Congpuong K, et al. Pharmacokinetics of oral artemether in Thai patients with uncomplicated falciparum malaria. Fundam Clin Pharmacol 1998; 12(2): 242–4
Karbwang J, Na-Bangchang K, Tin T, et al. Pharmacokinetics of intramuscular artemether in patients with severe falciparum malaria with or without acute renal failure. Br J Clin Pharmacol 1998 Jun; 45(6): 597–600
Karbwang J, Na-Bangchang K, Congpuong K, et al. Pharmacokinetics and bioavailability of oral and intramuscular artemether. Eur J Clin Pharmacol 1997; 52(4): 307–10
Batty KT, Le AT, Ilett KF, et al. A pharmacokinetic and pharmacodynamic study of artesunate for vivax malaria. Am J Trop Med Hyg 1998 Nov; 59(5): 823–7
Fitzsimmons ME, Collins JM. Selective biotransformation of the human immunodeficiency virus protease inhibitor saquinavir by human small-intestinal cytochrome P4503A4: potential contribution to high first-pass metabolism. Drug Metab Dispos 1997 Feb; 25(2): 256–66
Gonzalez FJ, Idle JR. Pharmacogenetic phenotyping and genotyping. Present status and future potential. Clin Pharmacokinet 1994 Jan; 26(1): 59–70
Wilkinson GR. Cytochrome P4503A (CYP3A) metabolism: prediction of in vivo activity in humans. J Pharmacokinet Biopharm 1996 Oct; 24(5): 475–90
Halliday RC, Jones BC, Smith DA, et al. An investigation of the interaction between halofantrine, CYP2D6 and CYP3A4: studies with human liver microsomes and heterologous enzyme expression systems. Br J Clin Pharmacol 1995 Oct; 40(4): 369–78
Zhao xJ, Yokoyama H, Chiba K, et al. Identification of human cytochrome P450 isoforms involved in the 3-hydroxylation of quinine by human liver microsomes and nine recombinant human cytochromes P450. J Pharmacol Exp Ther 1996 Dec; 279(3): 1327–34
Grace JM, Aguilar AJ, Trotman KM, et al. Metabolism of beta-arteether to dihydroqinghaosu by human liver microsomes and recombinant cytochrome P450. Drug Metab Dispos 1998 Apr; 26(4): 313–7
Bangchang KN, Karbwang J, Back DJ. Mefloquine metabolism by human liver microsomes. Effect of other antimalarial drugs. Biochem Pharmacol 1992 May 8; 43(9): 1957–61
Riviere JH, Back DJ. Inhibition of ethinylestradiol and tolbutamide metabolism by quinoline derivates in vitro. Chem Biol Interact 1986 Oct 1; 59(3): 301–8
Lefèvre G, Bindschedler M, Ezzet F, et al. Pharmacokinetic interaction trial between co-artemether and mefloquine. Eur J Pharma Sci. In press
Bakshi R, Hermeling-Fritz I, Gathmann I, et al. An integrated review of the clinical safety of coartemether (Riamet®): a novel oral antimalarial drug [abstract no. C14]. 6th Conference of the International Society of Travel Medicine; 1999 Jun 6–10; Montreal, Quebec, Canada
Svensson USH, Ashton M, Hai TN, et al. Artemisinin induces omeprazole metabolism in human beings. Clin Pharmacol Ther 1998 Aug; 64(2): 160–7
Ashton M, Hai TN, Sy ND, et al. Artemisinin pharmacokinetics is time-dependent during repeated oral administration in healthy male adults. Drug Metab Dispos 1998 Jan; 26(1): 25–7
Sidhu JS, Ashton M, Huong NV, et al. Artemisinin population pharmacokinetics in children and adults with uncomplicated falciparum malaria. Br J Clin Pharmacol 1998 Apr; 45(4): 347–54
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lefèvre, G., Thomsen, M.S. Clinical Pharmacokinetics of Artemether and Lumefantrine (Riamet®). Clin. Drug Investig. 18, 467–480 (1999). https://doi.org/10.2165/00044011-199918060-00006
Published:
Issue Date:
DOI: https://doi.org/10.2165/00044011-199918060-00006