Abstract
Objective: This study aimed too compare the pharmacokinetic and pharmacodynamic profile of intermittent and continuously infused ceftazidime in patients with nosocomial pneumonia.
Design and Setting: Prospective, randomised study set in a large community-teaching hospital.
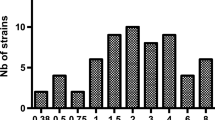
Interventions: Thirty-four patients receiving ceftazidime either as an intermittent infusion or a continuous infusion underwent blood sampling for drug concentration determinations between days 2 to 5 of therapy. In addition, at study enrolment sputum samples were obtained for Gram’s stain and culture. The minimum inhibitory concentration (MIC) of isolated organisms to ceftazidime was determined using the broth microdilution technique.
Main Outcome Measures: Pharmacokinetic parameters were derived individually for each patient. The pharmacodynamic profile of ceftazidime was assessed as the duration of time the serum concentration remained above the MIC (T > MIC) of the organism(s) for each regimen.
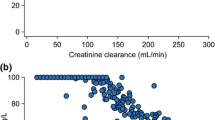
Results: Patients were well matched for demographic variables. The pharmacokinetic parameter estimates (mean ± SD) for patients receiving the intermittent infusion therapy were as follows: maximum concentration in serum 106.5 ± 34.6 mg/L; half-life 3.2 ± 2.5 hours; total body clearance (CLt) 142.5 ± 59 ml/min. The steady-state concentration with the continuous infusion regimen was 17.4 ± 6.1 mg/L, while the CLt was similar at 133.2 ± 37 ml/min. Forty-six pathogens were isolated in 27 patients. The continuous infusion regimen maximised the pharmacodynamics of ceftazidime as T > MIC was 100% of the dosage interval in all patients, whereas the intermittent infusion regimens resulted in T > MIC values of 56 to 100%.
Conclusions: In critically ill patients with nosocomial pneumonia the administration of ceftazidime by continuous infusion appears to optimise the pharmacodynamic profile of this β-lactam by constantly providing concentrations in excess of the MIC of susceptible organisms over the course of therapy.
Similar content being viewed by others
References
Craig WA. Interrelationships between pharmacokinetic and pharmacodynamics in determining dosage regimens for broad-spectrum cephalosporins. Diagn Microbiol Infect Dis 1995; 22: 89–96
Nicolau DP, Lacy MK, McNabb JC, et al. Pharmacokinetics of continuous and intermittent ceftazidime in intensive care unit patients with nosocomial pneumonia. Infect Dis Clin Prac 1999; 8: 45–9
Garner JS, Jarvis WR, Emori TG, et al. CDC definitions for nosocomial infections, 1988. Am J Infect Control 1988; 16: 128–40
Nicolau DP, Freeman CD, Belliveau PP, et al. Experience with a once-daily aminoglycoside program administered to 2,184 adult patients. Antimicrob Agents Chemother 1995; 39:650–5
Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron 1976; 16: 31–41
Nicolau DP, Nightingale CH, Banevicius MA, et al. Ceftazidime serum bactericidal activity: continuous infusion versus intermittent injections. Antimicrob Agents Chemother 1996; 40: 61–4
National Committee for Clinical Laboratory Standards: methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved Standard. NCCLS document M7-A4. Wayne, National Committee for Clinical Laboratory Standards, 1997
Eagle H, Fleischman R, Levy M. Continuous vs. discontinuous therapy with penicillin. N Engl J Med 1953; 248: 481–8
Craig WA, Ebert SC. Continuous infusion of β-lactam anti-biotics. Antimicrob Agents Chemother 1992; 36: 2577–83
Vondracek TG. Beta-lactam antibiotics: is continuous infusion the preferred method of administration? Ann Pharmacother 1995; 29: 415–24
Bodey GP, Ketchel SJ, Rodriquez V. A randomized study of carbenicillin plus cefamandole or tobramycin in the treatment of febrile episodes in cancer patients. Am J Med 1979; 67: 608–11
Zeisler JA, McCarthy JD, Richelieu WA, et al. Cefuroxime by continuous infusion: a new standard of care? Infect Med 1992; 11:54–60
Ambrose PG, Quintiliani R, Nightingale CH, et al. Continuous versus intermittent infusion of cefuroxime for the therapy of community-acquired pneumonia. Infect Dis Clin Prac 1998; 7: 463–70
Daenen S, Erjavec Z, Uges DRA, et al. Continuous infusion of ceftazidime in febrile neutropenic patients with acute myeloid leukemia. Eur J Clin Microbiol Infect Dis 1995; 14: 188–92
Houlihan HH, Mercier RC, McKinnon PS, et al. Continuous infusion versus intermittent administration of ceftazidime in critically ill patients with Gram-negative infection. [abstract No. A-42). 37th Interscience Conference on Antimicrobial Agents and Chemotherapy; 1997 Sep 28-Octl: Toronto, Ontario, Canada
Benko AS, Cappelletty DM, Kruse JA, et al. Continuous infusion versus intermittent administration of ceftazidime in critically ill patients with suspected Gram-negative infections. Antimicrob Agents Chemother 1996; 40: 691–5
Castela N, Taburet AM, Carlet J, et al. Pharmacokinetics of ceftazidime during continuous infusion in intensive care patients [abstract No. A11]. Program Abstracts 34th Inter-science Conference on Antimicrobial Agents and Chemotherapy; 1994 Oct 4–7: Orlando, Florida
Young RJ, Lipman J, Gin T, et al. Intermittent bolus dosing of ceftazidime in critically ill patients. J Antimicrob Chemother 1997; 40: 269–73
Vinks AATMM, Brimicombe RW, Heijerman et al. Continuous infusion of ceftazidime in cystic fibrosis patients during home treatment: clinical outcome microbiology, and pharmacokinetics. J Antimicrob Chemother 1997; 40: 125–33
National Committee for Clinical and Laboratory Standards. Performance standards for antimicrobial susceptibility testing; Eighth informational supplement. NCCLS document M100-S8. Wayne, PA. 1998
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nicolau, D.P., McNabb, J., Lacy, M.K. et al. Pharmacokinetics and Pharmacodynamics of Continuous and Intermittent Ceftazidime during the Treatment of Nosocomial Pneumonia. Clin. Drug Investig. 18, 133–139 (1999). https://doi.org/10.2165/00044011-199918020-00006
Published:
Issue Date:
DOI: https://doi.org/10.2165/00044011-199918020-00006