Summary
Abstract
Extended-release ranolazine (ranolazine ER) [Ranexa®] is a piperazine derivative with a novel mechanism of action that was recently approved in the EU for use as add-on therapy in patients with stable angina pectoris. Ranolazine ER achieves its antianginal effect without affecting heart rate or blood pressure (BP) to a clinically significant extent. Results of well designed, placebo-controlled, short-term studies demonstrate that add-on therapy with ranolazine ER in patients with chronic stable angina improves exercise performance, and reduces anginal frequency and nitroglycerin use. Although longer-term therapy with ranolazine ER did not reduce the incidence of major cardiovascular events in patients with non-ST-elevation acute coronary syndromes, it did reduce the incidence of recurrent ischaemia. Ranolazine ER is a generally well tolerated antianginal agent. Although it is associated with modest dose-related increases in the corrected QT (QTc) interval, ranolazine ER does not appear to be associated with an excess of arrhythmias. Thus, ranolazine ER is a useful new option for patients with chronic stable angina whose symptoms are not controlled with first-line antianginal therapy or who do not tolerate first-line antianginal agents.
Pharmacological Properties
The proposed mechanism of action for ranolazine is inhibition of the late inward sodium current in cardiac cells. Ranolazine also inhibits the rapidly activating component of the delayed rectifier potassium current (IKr). Ranolazine has antianginal activity, but does not affect heart rate or BP to a clinically significant extent. Despite modestly increasing the QTc interval, ranolazine appears to lack the proarrhythmic activity typically associated with drugs that inhibit IKr and even demonstrated antiarrhythmic activity in preclinical and clinical trials. Ranolazine ER improved glycaemic control in patients with diabetes mellitus and chronic stable angina or non-ST-elevation acute coronary syndromes.
Steady-state pharmacokinetics are usually reached within 3 days with twice-daily administration of ranolazine ER. The drug is primarily metabolized by cytochrome P450 (CYP) 3A, with a lesser contribution from CYP2D6. In the EU, contraindications to ranolazine ER include its use in patients with moderate or severe hepatic impairment and its concomitant administration with potent CYP3A inhibitors.
Therapeutic Efficacy
The efficacy of ranolazine ER in patients with chronic stable angina was examined in three randomized, double-blind, placebo-controlled, multicentre trials of up to 12 weeks' duration: the crossover MARISA trial (examining ranolazine ER monotherapy) and the parallel-group CARISA and ERICA trials (examining addon therapy with ranolazine ER).
In the MARISA trial, monotherapy with ranolazine ER 500, 1000 or 1500 mg twice daily increased the total exercise duration at trough (primary endpoint) and peak drug concentrations, the time to onset of angina at trough and peak, and the time to 1 mm ST-segment depression at trough and peak to a significantly greater extent than placebo.
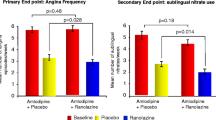
In the CARISA trial, the addition of ranolazine ER 750 or 1000 mg twice daily to background therapy comprising atenolol, diltiazem or amlodipine increased exercise duration at trough (primary endpoint) and peak, and the time to onset of angina at trough and peak to a significantly greater extent than placebo. The time to 1 mm ST-segment depression was increased to a significantly greater extent with ranolazine ER 750 or 1000 mg twice daily than with placebo only at peak ranolazine concentrations. Angina frequency and nitroglycerin use were significantly lower with ranolazine ER 750 or 1000 mg twice daily than with placebo.
In the ERICA trial, angina frequency (primary endpoint) and nitroglycerin use were significantly lower with ranolazine ER 1000 mg twice daily plus amlodipine than with placebo plus amlodipine. The mean Seattle Angina Questionnaire score for the angina frequency dimension improved to a significantly greater extent with ranolazine ER 1000 mg twice daily than with placebo.
MERLIN-TIMI 36 was a randomized, double-blind, placebo-controlled, multinational trial examining the efficacy of ranolazine ER in patients with non-ST-elevation acute coronary syndromes who were at moderate to high risk of death or recurrent ischaemic events (median duration of follow-up 348 days). The incidence of the composite primary endpoint (cardiovascular death, myocardial infarction or recurrent ischaemia) did not significantly differ between ranolazine ER and placebo recipients at study end. However, patients receiving ranolazine ER were significantly less likely than those receiving placebo to experience recurrent ischaemia.
Tolerability
Ranolazine ER was generally well tolerated in short-term trials (CARISA, MARISA, ERICA) and in the longer-term ROLE extension trial (mean duration of follow-up 2.8 years) in patients with chronic stable angina. The most commonly occurring adverse events included dizziness, nausea, asthenia and constipation. In the MARISA, CARISA and ROLE trials, ranolazine ER was associated with modest dose-related increases in the QTc interval.
In the MERLIN-TIMI 36 trial, there was no significant difference between patients receiving ranolazine ER and those receiving placebo in the incidence of death from any cause at months 6, 12 or 18; death or any cardiovascular hospitalization; sudden cardiac death; or symptomatic documented arrhythmia. In addition, the incidence of clinically significant arrhythmias during Holter monitoring over the first 7 days of the trial was significantly lower in ranolazine ER than in placebo recipients.




Similar content being viewed by others
Notes
The use of trade names is for product identification purposes only and does not imply endorsement.
References
Allender S, Scarborough P, Peto V, et al. European cardiovascular disease statistics: 2008 edition [online]. Available from URL: http://www.ehnheart.org/files/statistics%202008%20web-161229A.pdf [Accessed 2008 Aug 12]
Fox K, Garcia MA, Ardissino D, et al. Guidelines on the management of stable angina pectoris: executive summary: the task force on the management of stable angina pectoris of the European Society of Cardiology. Eur Heart J 2006 Jun; 27(11): 1341–81
Scirica BM, Morrow DA. Ranolazine in patients with angina and coronary artery disease. Curr Cardiol Rep 2007 Jul; 9(4): 272–8
European Medicines Agency. Ranexa (ranolazine): EU summary of product characteristics [online]. Available from URL: http://www.emea.europa.eu[Accessed 2008 Aug 5]
Dobesh PP, Trujillo TC. Ranolazine: a new option in the management of chronic stable angina. Pharmacotherapy 2007 Dec; 27(12): 1659–76
Jerling M. Clinical pharmacokinetics of ranolazine. Clin Pharmacokinet 2006; 45(5): 469–91
Bassand J-P. Clinical implications of inhibition of the late sodium current: ranolazine. Eur Heart J Suppls 2006; 8 Suppl. A: A14–9
Saint DA. The cardiac persistent sodium current: an appealing therapeutic target? Br J Pharmacol 2008 Mar; 153(6): 1133–42
Shryock JC, Belardinelli L. Inhibition of late sodium current to reduce electrical and mechanical dysfunction of ischaemic myocardium. Br J Pharmacol 2008 Mar; 153(6): 1128–32
Hale SL, Shryock JC, Belardinelli L, et al. Late sodium current inhibition as a new cardioprotective approach. J Mol Cell Cardiol 2008 Jun; 44(6): 954–67
Fredj S, Sampson KJ, Liu H, et al. Molecular basis of ranolazine block of LQT-3 mutant sodium channels: evidence for site of action. Br J Pharmacol 2006 May; 148(1): 16–24
Antzelevitch C, Belardinelli L, Zygmunt AC, et al. Electrophysiological effects of ranolazine, a novel antianginal agent with antiarrhythmic properties. Circulation 2004 Aug 24; 110(8): 904–10
Undrovinas AI, Belardinelli L, Undrovinas NA, et al. Ranolazine improves abnormal repolarization and contraction in left ventricular myocytes of dogs with heart failure by inhibiting late sodium current. J Cardiovasc Electrophysiol 2006 May; 17 Suppl. 1: S169–77
Sossalla S, Wagner S, Rasenack ECL, et al. Ranolazine improves diastolic dysfunction in isolated myocardium from failing human hearts: role of late sodium current and intracellular ion accumulation. J Mol Cell Cardiol 2008 Jul; 45(1): 32–43
Song Y, Shryock JC, Wagner S, et al. Blocking late sodium current reduces hydrogen peroxide-induced arrhythmogenic activity and contractile dysfunction. J Pharmacol Exp Ther 2006; 318(1): 214–22
Song Y, Shryock JC, Wu L, et al. Antagonism by ranolazine of the pro-arrhythmic effects of increasing late INa in guinea pig ventricular myocytes. J Cardiovascul Pharmacol 2004 Aug; 44(2): 192–9
Wu L, Shryock JC, Song Y, et al. An increase in late sodium current potentiates the proarrhythmic activities of low-risk QT-prolonging drugs in female rabbit hearts. J Pharmacol Exp Ther 2006; 316(2): 718–26
Wu L, Shryock JC, Song Y, et al. Antiarrhythmic effects of ranolazine in a guinea pig in vitro model of long-QT syndrome. J Pharmacol Exp Ther 2004; 310(2): 599–605
Moss AJ, Zareba W, Schwarz KQ, et al. Ranolazine shortens repolarization in patients with sustained inward sodium current due to type-3 long-QT syndrome. J Cardiovasc Electrophysiol. Epub 2008 Jul 25
Fraser H, Belardinelli L, Wang L, et al. Ranolazine decreases diastolic calcium accumulation caused by ATX-II or ischemia in rat hearts. J Mol Cell Cardiol 2006 Dec; 41(6): 1031–8
Clarke B, Wyatt KM, McCormack JG. Ranolazine increases active pyruvate dehydrogenase in perfused normoxic rat hearts: evidence for an indirect mechanism. J Mol Cell Cardiol 1996 Feb; 28(2): 341–50
McCormack JG, Barr RL, Wolff AA, et al. Ranolazine stimulates glucose oxidation in normoxic, ischemic, and reperfused ischemic rat hearts. Circulation 1996 Jan 1; 93(1): 135–42
MacInnes A, Fairman DA, Binding P, et al. The antianginal agent trimetazidine does not exert its functional benefit via inhibition of mitochondrial long-chain 3-ketoacyl coenzyme A thiolase. Circ Res 2003 Aug 8; 93(3): e26–32
Wang P, Fraser H, Lloyd SG, et al. A comparison between ranolazine and CVT-4325, a novel inhibitor of fatty acid oxidation, on cardiac metabolism and left ventricular function in rat isolated perfused heart during ischemia and reperfusion. J Pharmacol Exp Ther 2007 Apr; 321(1): 213–20
Létienne R, Vié B, Puech A, et al. Evidence that ranolazine behaves as a weak β1- and β2-adrenoceptor antagonist in the rat cardiovascular system. Naunyn Schmiedebergs Arch Pharmacol 2001 Apr; 363(4): 464–71
Bagger JP, Bøtker HE, Thomassen A, et al. Effects of ranolazine on ischemic threshold, coronary sinus blood flow, and myocardial metabolism in coronary artery disease. Cardiovasc Drugs Ther 1997 Jul; 11(3): 479–84
Chaitman BR, Skettino SL, Parker JO, et al. Anti-ischemic effects and long-term survival during ranolazine monotherapy in patients with chronic severe angina. J Am Coll Cardiol 2004 Apr 21; 43(8): 1375–82
Chaitman BR, Pepine CJ, Parker JO, et al. Effects of ranolazine with atenolol, amlodipine, or diltiazem on exercise tolerance and angina frequency in patients with severe chronic angina: a randomized controlled trial. JAMA 2004 Jan 21; 291(3): 309–16
Cocco G, Rousseau MF, Bouvy T, et al. Effects of a new metabolic modulator, ranolazine, on exercise tolerance in angina pectoris patients treated with β-blocker or diltiazem. J Cardiovasc Pharmacol 1992 Jul; 20(1): 131–8
Jain D, Dasgupta P, Hughes LO, et al. Ranolazine (RS-43285): a preliminary study of a new anti-anginal agent with selective effect on ischaemic myocardium. Eur J Clin Pharmacol 1990; 38(2): 111–4
Pepine CJ, Wolff AA. A controlled trial with a novel anti-ischemic agent, ranolazine, in chronic stable angina pectoris that is responsive to conventional antianginal agents. Ranolazine Study Group. Am J Cardiol 1999 Jul 1; 84(1): 46–50
Rousseau MF, Pouleur H, Cocco G, et al. Comparative efficacy of ranolazine versus atenolol for chronic angina pectoris. Am J Cardiol 2005 Feb 1; 95(3): 311–6
Stone PH, Gratsiansky NA, Blokhin A, et al. Antianginal efficacy of ranolazine when added to treatment with amlodipine: the ERICA (Efficacy of Ranolazine in Chronic Angina) trial. J Am Coll Cardiol 2006 Aug 1; 48(3): 566–75
Thadani U, Ezekowitz M, Fenney L, et al. Double-blind efficacy and safety study of a novel anti-ischemic agent, ranolazine, versus placebo in patients with chronic stable angina pectoris. Circulation 1994 Aug; 90(2): 726–34
Hayashida W, van Eyll C, Rousseau MF, et al. Effects of ranolazine on left ventricular regional diastolic function in patients with ischemic heart disease. Cardiovasc Drugs Ther 1994 Oct; 8(5): 741–7
Stone PH, Chaitman B, Koren A, et al. Effects of ranolazine as monotherapy and combination therapy on rate pressure product at rest and during exercise: results from the MARISA and CARISA trials [abstract no. 3362]. Circulation 2006 Oct 31; 114 (18 Suppl.): 715
Rajamani S, Shryock JC, Belardinelli L. Rapid kinetic interactions of ranolazine with HERG K+ current. J Cardiovasc Pharmacol 2008 Jun; 51(6): 581–9
Kumar K, Nearing BD, Bartoli CR, et al. Effect of ranolazine on ventricular vulnerability and defibrillation threshold in the intact porcine heart. J Cardiovasc Electrophysiol 2008 Oct; 19(10): 1073–9
Burashnikov A, Di Diego JM, Zygmunt AC, et al. Atriumselective sodium channel block as a strategy for suppression of atrial fibrillation: differences in sodium channel inactivation between atria and ventricles and the role of ranolazine. Circulation 2007 Sep 25; 116(13): 1449–57
Schram G, Zhang L, Derakhchan K, et al. Ranolazine: ion-channel-blocking actions and in vivo electrophysiological effects. Br J Pharmacol 2004; 142(8): 1300–8
Wang W-Q, Robertson C, Dhalla AK, et al. Antitorsadogenic effects of (±)-N-(2,6-dimethyl-phenyl)-(4[2-hydroxy-3-(2-methoxyphenoxy)propyl]-1-piperazine (ranolazine) in anesthetized rabbits. J Pharmacol Exp Ther 2008 Jun; 325(3): 875–81
Gralinski MR, Chi L, Park JL, et al. Protective effects of ranolazine on ventricular fibrillation induced by activation of the ATP-dependent potassium channel in the rabbit heart. J Cardiovasc Pharmacol Ther 1996 Apr; 1(2): 141–8
Matsumura H, Hara A, Hashizume H, et al. Protective effects of ranolazine, a novel anti-ischemic drug, on the hydrogen peroxide-induced derangements in isolated, perfused rat heart: comparison with dichloroacetate. Jpn J Pharmacol 1998 May; 77(1): 31–9
Gralinski MR, Black SC, Kilgore KS, et al. Cardioprotective effects of ranolazine (RS-43285) in the isolated perfused rabbit heart. Cardiovasc Res 1994 Aug; 28(8): 1231–7
Chandler MP, Stanley WC, Morita H, et al. Short-term treatment with ranolazine improves mechanical efficiency in dogs with chronic heart failure. Circ Res 2002 Aug 23; 91(4): 278–80
Sabbah HN, Chandler MP, Mishima T, et al. Ranolazine, a partial fatty acid oxidation (pFOX) inhibitor, improves left ventricular function in dogs with chronic heart failure. J Card Fail 2002 Dec; 8(6): 416–22
Timmis AD, Chaitman BR, Crager M. Effects of ranolazine on exercise tolerance and HbA1c in patients with chronic angina and diabetes. Eur Heart J 2006; 27(1): 42–8
Morrow DA, Scirica BM, Chaitman BR, et al. Effect of ranolazine on hemoglobin A1c in the MERLIN-TIMI 36 randomized controlled trial [abstract no. 2453]. Circulation 2007 Oct 16; 116 (16 Suppl. II): 539–40
Morrow DA, Scirica BM, Karwatowska-Prokopczuk E, et al. Effects of ranolazine on recurrent cardiovascular events in patients with non-ST-elevation acute coronary syndromes: the MERLIN-TIMI 36 randomized trial. JAMA 2007 Apr 25; 297(16): 1775–83
CV Therapeutics, Inc. Ranexa® (ranolazine extended-release tablets): US prescribing information [online]. Available from URL: http://www.cvt.com/pdf/Ranexa%20PI%20L000025%201108.pdf[Accessed 2008 Jul 23]
Penman AD, Eadie J, Herron WJ, et al. The characterization of the metabolites of ranolazine in man by liquid chromatography mass spectrometry. Rapid Commun Mass Spectrom 1995; 9(14): 1418–30
Chu N, Sohoo D, Sun H-L, et al. In vitro metabolism of ranolazine [abstract no. 363]. Drug Metab Rev 2003; 35 Suppl. 2: 182
Jerling M, Huan B-L, Leung K, et al. Studies to investigate the pharmacokinetic interactions between ranolazine and ketoconazole, diltiazem, or simvastatin during combined administration in healthy subjects. J Clin Pharmacol 2005 Apr; 45(4): 422–33
Chu N, Lustig D, Wong S, et al. Disposition of [14C]-ranolazine in humans [abstract no. 98]. Drug Metab Rev 2003; 35 Suppl. 2: 49
Abdallah H, Jerling M. Effect of hepatic impairment on the multiple-dose pharmacokinetics of ranolazine sustained-release tablets. J Clin Pharmacol 2005 Jul; 45(7): 802–9
Jerling M, Abdallah H. Effect of renal impairment on multiple-dose pharmacokinetics of extended-release ranolazine. Clin Pharmacol Ther 2005 Sep; 78(3): 288–97
Parker J, Chaitman B, Skopal J, et al. Rebound worsening in exercise performance was not observed after abrupt ranolazine withdrawal in patients with chronic angina in CARISA [abstract no. 188]. Eur Heart J 2003; 24 Suppl. 1: 20
Rich MW, Crager M, McKay CR. Safety and efficacy of extended-release ranolazine in patients aged 70 years or older with chronic stable angina pectoris. Am J Geriatr Cardiol 2007; 16(4): 216–21
Wenger NK, Chaitman B, Vetrovec GW. Gender comparison of efficacy and safety of ranolazine for chronic angina pectoris in four randomized clinical trials. Am J Cardiol 2007 Jan 1; 99(1): 11–8
White HD, Skettino S, Chaitman BR, et al. Anti-anginal efficacy of ranolazine addition to beta blocker or calcium antagonist therapy in patients with a history of heart failure [abstract no. 1746]. Circulation 2002 Nov 5; 106 (19 Suppl. II): 349–50
European Medicines Agency. CHMP assessement report for Ranexa (ranolazine) [online]. Available from URL: http://www.emea.europa.eu[Accessed 2008 Aug 5]
Morrow DA, Scirica BM, Karwatowska-Prokopczuk E, et al. Evaluation of a novel anti-ischemic agent in acute coronary syndromes: design and rationale for the Metabolic Efficiency with Ranolazine for Less Ischemia in Non-ST-elevation acute coronary syndromes (MERLIN)-TIMI 36 trial. Am Heart J 2006 Aug; 152(2): 400e.1-9
Arnold SV, Morrow DA, Wang K, et al. Effects of ranolazine on disease-specific health status and quality of life: results from the MERLIN-TIMI 36 randomized trial [abstract no. 1024-54]. J Am Coll Cardiol 2008 Mar 11; 51 (10 Suppl. A): A215
Mega JL, Hochman JS, Scirica BM, et al. Anti-ischemic effects of ranolazine in women: results from the randomized, placebocontrolled MERLIN-TIMI 36 trial [abstract no. 2451]. Circulation 2007 Oct 16; 116 (16 Suppl. II): 539
Wilson SR, Morrow DA, Scirica BM, et al. Efficacy and safety of ranolazine in chronic angina: observations from the randomized, double-blind, placebo-controlled MERLIN-TIMI 36 Trial [abstract no. 1031-45]. J Am Coll Cardiol 2008 Mar 11; 51 (10 Suppl. A): A225
Koren MJ, Crager MR, Sweeney M. Long-term safety of a novel antianginal agent in patients with severe chronic stable angina: the Ranolazine Open Label Experience (ROLE). J Am Coll Cardiol 2007 Mar 13; 49(10): 1027–34
Scirica BM, Morrow DA, Hod H, et al. Effect of ranolazine, an antianginal agent with novel electrophysiological properties, on the incidence of arrhythmias in patients with non ST-segment elevation acute coronary syndrome: results from the Metabolic Efficiency with Ranolazine for Less Ischemia in Non ST-Elevation Acute Coronary Syndrome Thrombolysis in Myocardial Infarction 36 (MERLIN-TIMI 36) randomized controlled trial. Circulation 2007 Oct 9; 116(15): 1647–52
Gibbons RJ, Abrams J, Chatterjee K, et al. ACC/AHA 2002 guideline update for the management of patients with chronic stable angina: a report of the American College of Cardiology/ American Heart Association Task Force on Practice Guidelines (committee to update the 1999 guidelines for the management of patients with chronic stable angina) [online]. Available from URL: http://www.acc.org/clinical/guidelines/stable/stable.pdf[Accessed 2008 Aug 5]
Boden WE, O'Rourke RA, Teo KK, et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med 2007 Apr 12; 356(15): 1503–16
Melloni C, Newby LK. Metabolic efficiency with ranolazine for less ischemia in non-ST elevation acute coronary syndromes (MERLIN TIMI-36) study. Expert Rev Cardiovasc Ther 2008 Jan; 6(1): 9–16
Newby LK, Peterson ED. Does ranolazine have a place in the treatment of acute coronary syndromes? JAMA 2007 Apr 25; 297(16): 1823–5
Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association task force on practice guidelines (writing committee to revise the 2002 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction). Circulation 2007 Aug 14; 116(7): e148–304
Chaitman BR. Ranolazine for the treatment of chronic angina and potential use in other cardiovascular conditions. Circulation 2006 May 23; 113(20): 2462–72
Cobbe S. Electrophysiological perspectives: what has ranolazine taught us? Eur Heart J Suppls 2004; 6 Suppl. 1: I9–I11
Author information
Authors and Affiliations
Corresponding author
Additional information
Various sections of the manuscript reviewed by: J. Abrams, Division of Cardiology, Department of Internal Medicine, The University of New Mexico Health Sciences Center, Albuquerque, New Mexico, USA; J-P. Bassand, Department of Cardiology, University Hospital Jean Minjoz, Besançon, France; G. Cocco, Cardiology Office, Rheinfelden, Switzerland; H. Hod, Intensive Cardiac Care Unit, Sheba Medical Center, Tel-Aviv University, Tel-Hashomer, Israel; L.S. Maier, Department of Cardiology and Pneumology, Georg-August-Universität Göttingen, Göttingen, Germany; C.J. Pepine, Division of Cardiovascular Medicine, University of Florida College of Medicine, Gainesville, Florida, USA; B.M. Scirica, Cardiovascular Division, Department of Medicine, Brigham and Women’s Hospital, Boston, Massachusetts, USA.
Data Selection
Sources: Medical literature published in any language since 1980 on ‘ranolazine’, identified using MEDLINE and EMBASE, supplemented by AdisBase (a proprietary database of Wolters Kluwer Health | Adis). Additional references were identified from the reference lists of published articles. Bibliographical information, including contributory unpublished data, was also requested from the company developing the drug.
Search strategy: MEDLINE, EMBASE and AdisBase search terms were ‘ranolazine’ and ‘angina pectoris’. Searches were last updated 20 October 2008.
Selection: Studies in patients with chronic stable angina pectoris who received ranolazine. Inclusion of studies was based mainly on the methods section of the trials. When available, large, well controlled trials with appropriate statistical methodology were preferred. Relevant pharmacodynamic and pharmacokinetic data are also included.
Index terms: Ranolazine, angina pectoris, pharmacodynamics, pharmacokinetics, therapeutic use, tolerability.
An erratum to this article is available at http://dx.doi.org/10.2165/11595790-000000000-00000.
Rights and permissions
About this article
Cite this article
Keating, G.M. Ranolazine. Drugs 68, 2483–2503 (2008). https://doi.org/10.2165/0003495-200868170-00006
Published:
Issue Date:
DOI: https://doi.org/10.2165/0003495-200868170-00006