Abstract
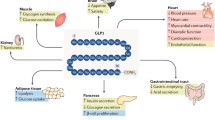
Glucagon-like peptide-1 (GLP-1) is synthesized from proglucagon in enteroendocrine cells and regulates glucose homeostasis via multiple complementary actions on appetite, gastrointestinal motility and islet hormone secretion. GLP-1 is secreted from the distal gut in response to food ingestion, and levels of circulating GLP-1 may be diminished in patients with type 2 diabetes mellitus. GLP-1 administration stimulates glucose-dependent insulin secretion, inhibits glucagon secretion, and lowers blood glucose in normal and diabetic rodents and in humans. GLP-1 exerts additional glucose-lowering actions in patients with diabetes mellitus already treated with metformin or sulfonylurea therapy. GLP-1 inhibits gastric emptying in healthy individuals and those with diabetes mellitus, and excess GLP-1 administration may cause nausea or vomiting in susceptible individuals. Chronic GLP-1 treatment of normal or diabetic rodents is associated with bodyweight loss and GLP-1 agonists transiently inhibit food intake and may prevent bodyweight gain in humans. The potential for GLP-1 therapy to prevent deterioration of β-cell function is exemplified by studies demonstrating that GLP-1 analogs stimulate proliferation and neogenesis of β-cells, leading to expansion of β-cell mass in diabetic rodents.
The rapid N-terminal inactivation of bioactive GLP-1 by dipeptidyl peptidase-IV (DPP-IV) limits the utility of the native peptide for the treatment of patients with diabetes mellitus, and has fostered the development of more potent and stable protease-resistant GLP-1 analogs which exhibit longer durations of action. The importance of DPP-IV for glucose control is illustrated by the phenotype of rodents with genetic inactivation of DPP-IV which exhibit reduced glycemic excursion and increased levels of circulating GLP-1 in vivo. Inhibitors of DPP-IV potentiate incretin action by preventing degradation of GLP-1 and glucose-dependent insulinotropic peptide, and lower blood glucose in normal rodents and in experimental models of diabetes mellitus. Hence, orally available DPP-IV inhibitors also represent a new class of therapeutic agents that enhance incretin action for the treatment of patients with type 2 diabetes mellitus.
Similar content being viewed by others
References
King H, Aubert RE, Herman WH. Global burden of diabetes, 1995–2025: prevalence, numerical estimates, and projections. Diabetes Care 1998; 21(9): 1414–31
Zhang BB, Moller DE. New approaches in the treatment of type 2 diabetes. Curr Opin Chem Biol 2000; 4(4): 461–7
Moller DE. New drug targets for type 2 diabetes and the metabolic syndrome. Nature 2001; 414(6865): 821–7
Dupre J, Ross SA, Watson D, et al. Stimulation of insulin secretion by gastric inhibitory polypeptide in man. J Clin Endocrinol Metab 1973; 37(5): 826–8
Pederson RA, Schubert HE, Brown JC. Gastric inhibitory polypeptide: its physiologic release and insulinotropic action in the dog. Diabetes 1975; 24(12): 1050–6
Schmidt WE, Siegel EG, Creutzfeldt W. Glucagon-like peptide-1 but not glucagon-like peptide-2 stimulates insulin release from isolated rat pancreatic islets. Diabetologia 1985; 28: 704–7
Kreymann B, Ghatei MA, Williams G, et al. Glucagon-like peptide-1 7-36: A physiological incretin in man. Lancet 1987; II: 1300–4
Mojsov S, Weir GC, Habener JF. Insulinotropin: glucagon-like peptide I (7–37) co-encoded in the glucagon gene is a potent stimulator of insulin release in the perfused rat pancreas. J Clin Invest 1987; 79: 616–9
Creutzfeldt W. The incretin concept today. Diabetologia 1979; 16(2): 75–85
Fehmann H-C, Goke R, Goke B. Cell and molecular biology of the incretin hormones glucagon-like peptide 1 and glucose-dependent releasing polypeptide. Endocr Rev 1995; 16: 390–410
Goke R, Wagner B, Fehmann HC, et al. Glucose-dependency of the insulin stimulatory effect of glucagon-like peptide-1 (7–36) amide on the rat pancreas. Res Exp Med (Berl) 1993; 193(2): 97–103
Elahi D, McAloon-Dyke M, Fukagawa NK, et al. The insulinotropic actions of glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (7–37) in normal and diabetic subjects. Regul Pept 1994; 51(1): 63–74
Nauck MA, Heimesaat MM, Orskov C, et al. Preserved incretin activity of glucagon-like peptide 1 [7-36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J Clin Invest 1993; 91: 301–7
Kieffer TJ, Habener JF. The glucagon-like peptides. Endocr Rev 1999; 20(6): 876–913
Drucker DJ. The glucagon-like peptides. Diabetes 1998; 47: 159–69
Holst JJ, Orskov C, Nielsen OV, et al. Truncated glucagon-like peptide I, an insulin-releasing hormone from the distal gut. FEBS Lett 1987; 211: 169–74
Drucker DJ, Philippe J, Mojsov S, et al. Glucagon-like peptide I stimulates insulin gene expression and increases cyclic AMP levels in a rat islet cell line. Proc Natl Acad Sci U S A 1987; 84: 3434–8
Fehmann H-C, Habener JF. Insulinotropic hormone glucagon-like peptide-I (7–37) stimulation of proinsulin gene expression and proinsulin biosynthesis in insulinoma bTC-1 cells. Endocrinology 1992; 130: 159–66
Scrocchi LA, Brown TJ, MacLusky N, et al. Glucose intolerance but normal satiety in mice with a null mutation in the glucagon-like peptide receptor gene. Nat Med 1996; 2: 1254–8
Kolligs F, Fehmann H-C, Goke R, et al. Reduction of the incretin effect in rats by the glucagon-like peptide 1 receptor antagonist exendin (9–39) amide. Diabetes 1995; 44: 16–9
Schirra J, Sturm K, Leicht P, et al. Exendin (9–39)amide is an antagonist of glucagon-like peptide-1 (7–36)amide in humans. J Clin Invest 1998; 101(7): 1421–30
Edwards CM, Todd JF, Mahmoudi M, et al. Glucagon-like peptide 1 has a physiological role in the control of postprandial glucose in humans: studies with the antagonist exendin 9–39. Diabetes 1999; 48(1): 86–93
Holz GG, Kuhtreiber WM, Habener JF. Pancreatic beta-cells are rendered glucose-competent by the insulinotropic hormone glucagon-like peptide-1 (7–37). Nature 1993; 361: 362–5
Byrne MM, Gliem K, Wank U, et al. Glucagon-like peptide 1 improves the ability of the beta-cell to sense and respond to glucose in subjects with impaired glucose tolerance. Diabetes 1998; 47(8): 1259–65
Dachicourt N, Serradas P, Bailbe D, et al. Glucagon-like peptide-1 (7–36)-amide confers glucose sensitivity to previously glucose-incompetent beta-cells in diabetic rats: in vivo and in vitro studies. J Endocrinol 1997; 155(2): 369–76
Fridolf T, Bottcher G, Sundler F, et al. GLP-1 and GLP-1 (7–36) amide: influences on basal and stimulated insulin and glucagon secretion in the mouse. Pancreas 1991; 6(2): 208–15
Orskov C, Holst JJ, Nielsen OV. Effect of truncated glucagon-like peptide-1 [proglucagon-(78–107) amide] on endocrine secretion from pig pancreas, antrum, and nonantral stomach. Endocrinology 1988; 123: 2009–13
Suzuki S, Kawai K, Ohashi S, et al. Comparison of the effects of various C-terminal and N-terminal fragment peptides of glucagon-like peptide-1 on insulin and glucagon release from the isolated perfused rat pancreas. Endocrinology 1989; 125(6): 3109–14
Komatsu R, Matsuyama T, Namba M, et al. Glucagonostatic and insulinotropic action of glucagonlike peptide I-(7–36)-amide. Diabetes 1989; 38: 902–5
Matsuyama T, Komatsu R, Namba M, et al. Glucagon-like peptide-1 (7–36 amide): a potent glucagonostatic and insulinotropic hormone. Diabetes Res Clin Pract 1988; 5(4): 281–4
Vilsboll T, Krarup T, Madsbad S, et al. No reactive hypoglycaemia in Type 2 diabetic patients after subcutaneous administration of GLP-1 and intravenous glucose. Diabet Med 2001; 18(2): 144–9
Nauck MA, Niedereichholz U, Ettler R, et al. Glucagon-like peptide 1 inhibition of gastric emptying outweighs its insulinotropic effects in healthy humans. Am J Physiol 1997; 273: E981–8
Wettergren A, Schjoldager B, Mortensen PE, et al. Truncated GLP-1 (proglucagon 78–107-amide) inhibits gastric and pancreatic functions in man. Dig Dis Sci 1993; 38: 665–73
Willms B, Werner J, Holst JJ, et al. Gastric emptying, glucose responses, and insulin secretion after a liquid test meal: effects of exogenous glucagon-like peptide-1 (GLP-1)-(7–36)amide in type 2 (non-insulin dependent) diabetic patients. J Clin Endocrinol Metab 1996; 81: 327–32
Schirra J, Katschinski M, Weidmann C, et al. Gastric emptying and release of incretin hormones after glucose ingestion in humans. J Clin Invest 1996; 97: 92–103
Naslund E, Bogefors J, Skogar S, et al. GLP-1 slows solid gastric emptying and inhibits insulin, glucagon, and PYY release in humans. Am J Physiol 1999; 277 (3 Pt 2): R910–6
Shimizu I, Hirota M, Ohboshi C, et al. Identification and localization of glucagon-like peptide-1 and its receptor in rat brain. Endocrinology 1987; 121: 1076–82
Uttenthal LO, Toledano A, Blazquez E. Autoradiographic localization of receptors for glucagon-like peptide-1 (7–36) amide in rat brain. Neuropeptides 1992; 21: 143–6
Larsen PJ, Tang-Christensen M, Holst JJ, et al. Distribution of glucagon-like peptide-1 and other preproglucagon-derived peptides in the rat hypothalamus and brainstem. Neuroscience 1997; 77: 257–70
Shughrue PJ, Lane MV, Merchenthaler I. Glucagon-like peptide-1 receptor (GLP1-R) mRNA in the rat hypothalamus. Endocrinology 1996; 137: 5159–62
Navarro M, Rodriguez de Fonseca F, Alvarez E, et al. Colocalization of glucagon-like peptide-1 (GLP-1) receptors, glucose transporter GLUT-2, and glucokinase mRNAs in rat hypothalamic cells: Evidence for a role of GLP-1 receptor agonists as an inhibitory signal for food and water intake. J Neurochem 1996; 67: 1982–91
Alvarez E, Roncero I, Chowen JA, et al. Expression of the glucagon-like peptide-1 receptor gene in rat brain. J Neurochem 1996; 66: 920–7
Merchenthaler I, Lane M, Shughrue P. Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J Comp Neurol 1999; 403(2): 261–80
Turton MD, O’Shea D, Gunn I, et al. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature 1996; 379: 69–72
Tang-Christensen M, Larsen PJ, et al. Central administration of GLP-1 (7–36) amide inhibits food and water intake in rats. Am J Physiol 1996; 271: R848–56
Donahey JCK, Van Dijk G, Woods SC, et al. Intraventricular GLP-1 reduces short-but not long-term food intake or body weight in lean and obese rats. Brain Res 1998; 779: 75–83
Meeran K, O’Shea D, Edwards CM, et al. Repeated intracerebroventricular administration of glucagon-like peptide-l-(7–36) amide or exendin-(9–39) alters body weight in the rat. Endocrinology 1999; 140(1): 244–50
McMahon LR, Wellman PJ. PVN infusion of GLP-l-(7–36) amide suppresses feeding but does not induce aversion or alter locomotion in rats. Am J Physiol 1998; 274: R23–9
Flint A, Raben A, Astrup A, et al. Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J Clin Invest 1998; 101: 515–20
Gutzwiller JP, Drewe J, Goke B, et al. Glucagon-like peptide-1 promotes satiety and reduces food intake in patients with diabetes mellitus type 2. Am J Physiol 1999;276(5 Pt 2): R1541–4
Toft-Nielsen MB, Madsbad S, Holst JJ. Continuous subcutaneous infusion of glucagon-like peptide 1 lowers plasma glucose and reduces appetite in type 2 diabetic patients. Diabetes Care 1999; 22(7): 1137–43
Naslund E, Barkeling B, King N, et al. Energy intake and appetite are suppressed by glucagon-like peptide-1 (GLP-1) in obese men. Int J Obes Relat Metab Disord 1999; 23(3): 304–11
D’Alessio DA, Prigeon RL, Ensinck JW. Enterai enhancement of glucose disposition by both insulin-dependent and insulin-independent processes: a physiological role of glucagon-like peptide I. Diabetes 1995; 44: 1433–7
Valverde I, Villanueva-Penacarrillo ML. In vitro insulinomimetic [corrected] effects of GLP-1 in liver, muscle and fat. Acta Physiol Scand 1996; 157(3): 359–60
Miki H, Namba M, Nishimura T, et al. Glucagon-like peptide-1 (7–36) amide enhances insulin-stimulated glucose uptake and decreases intracellular cAMP content in isolated rat adipocytes. Biochim Biophys Acta 1996; 1312: 132–6
Morales M, Lopez-Delgado MI, Alcantara A, et al. Preserved GLP-1 effects on glycogen synthase a activity and glucose metabolism in isolated hepatocytes and skeletal muscle from diabetic rats. Diabetes 1997; 46: 1264–9
Blackmore PF, Mojsov S, Exton JH, et al. Absence of insulinotropic glucagon-like peptide-I (7–37) receptors on isolated rat liver hepatocytes. FEBS Lett 1991; 283: 7–10
Valverde I, Merida E, Delgado E, et al. Presence and characterization of glucagon-like peptide-1 (7–36) amide receptors in solubilized membranes of rat adipose tissue. Endocrinology 1993; 132: 75–9
Wheeler MB, Lu M, Dillon JS, et al. Functional expression of the rat glucagon-like peptide-I receptor, evidence for coupling to both adenylyl cyclase and phos-pholipase-C. Endocrinology 1993; 133: 57–62
Campos RV, Zhang L, Drucker DJ. Differential expression of RNA transcripts encoding unique carboxy-terminal sequences of human parathyroid-related peptide. Mol Endocrinol 1994; 9: 1656–66
Bullock BP, Heller RS, Habener JF.Tissue distribution of messenger ribonucleic acid encoding the rat glucagon-like peptide 1 receptor. Endocrinology 1996; 137: 2968–78
Sandhu H, Wiesenthal SR, MacDonald PE, et al. Glucagon-like peptide 1 increases insulin sensitivity in depancreatized dogs. Diabetes 1999; 48(5): 1045–53
OrskovL, Holst JJ, Moller J, et al. GLP-1 does not acutely affect insulin sensitivity in healthy man. Diabetologia 1996; 39: 1227–32
Ahren B, Larsson H, Holst JJ. Effects of glucagon-like peptide-1 on islet function and insulin sensitivity in noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab 1997; 82: 473–8
Vella A, Shah P, Basu R, et al. Effect of glucagon-like peptide 1 (7–36) amide on glucose effectiveness and insulin action in people with type 2 diabetes. Diabetes 2000; 49(4): 611–7
Burcelin R, Da Costa A, Drucker D, et al. Glucose competence of the hepatoportal vein sensor requires the presence of an activated glucagon-like peptide-1 receptor. Diabetes 2001; 50(8): 1720–8
Edvell A, Lindstrom P. Initiation of increased pancreatic islet growth in young normoglycemic mice (Umea +/?.). Endocrinology 1999; 140(2): 778–83
Susini S, Roche E, Prentki M, et al. Glucose and glucoincretin peptides synergize to induce c-fos, c-jun, junB, zif-268, and nur-77 gene expression in pancreatic beta (INS-1) cells. FASEB J 1998; 12(12): 1173–82
Buteau J, Roduit R, Susini S, et al. Glucagon-like peptide-1 promotes DNA synthesis, activates phosphatidylinositol 3-kinase and increases transcription factor pancreatic and duodenal homeobox gene 1 (PDX-1) DNA binding activity in beta (INS-l)-cells. Diabetologia 1999; 42(7): 856–64
Buteau J, Foisy S, Rhodes CJ, et al. Protein kinase Czeta activation mediates glucagon-like peptide-1-induced pancreatic beta-cell proliferation. Diabetes 2001; 50(10): 2237–43
Zhou J, Wang X, Pineyro MA, et al. Glucagon-like peptide 1 and exendin-4 convert pancreatic AR42J cells into glucagon- and insulin-producing cells. Diabetes 1999; 48(12): 2358–66
Hui H, Wright C, Perfetti R. Glucagon-like peptide 1 induces differentiation of islet duodenal homeobox-1-positive pancreatic ductal cells into insulin-secreting cells. Diabetes 2001; 50: 785–96
Xu G, Stoffers DA, Habener JF, et al. Exendin-4 stimulates both beta-cell replication and neogenesis, resulting in increased beta-cell mass and improved glucose tolerance in diabetic rats. Diabetes 1999; 48(12): 2270–6
Stoffers DA, Kieffer TJ, Hussain MA, et al. Insulinotropic glucagon-like peptide-1 agonists stimulate expression of homeodomain protein IDX-1 and increase β-cell mass in mouse pancreas. Diabetes 2000; 49: 741–8
Perfetti R, Zhou J, Doyle ME, et al. Glucagon-like peptide-1 induces cell proliferation and pancreatic-duodenum homeobox-1 expression and increases endocrine cell mass in the pancreas of old, glucose-intolerant rats. Endocrinology 2000; 141(12): 4600–5
Tourrel C, Bailbe D, Meile M-J, et al. Glucagon-like peptide-1 and exendin-4 stimulates b-cell neogenesis in streptozotocin-treated newborn rats resulting in persistently improved glucose homeostasis at adult age. Diabetes 2001; 50: 1562–70
Ling Z, Wu D, Zambre Y, et al. Glucagon-like peptide 1 receptor signaling influences topography of islet cells in mice. Virchows Arch 2001; 438(4): 382–7
Nauck MA, Kleine N, Orskov C, et al. Normalization of fasting hyperglycaemia by exogenous glucagon-like peptide 1 (7–36 amide) in Type 2 (non-insulin-dependent) diabetic patients. Diabetologia 1993; 36: 741–4
Rachman J, Gribble FM, Barrow BA, et al. Normalization of insulin responses to glucose by overnight infusion of glucagon-like peptide 1 (7–36) amide in patients with NIDDM. Diabetes 1996; 45: 1524–30
Rachman J, Barrow BA, Levy JC, et al. Near normalization of diurnal glucose concentrations by continuous administration of glucagon-like peptide 1 (GLP-1) in subjects with NIDDM. Diabetologia 1997; 40: 205–11
Gutniak MK, Linde B, Holst JJ, et al. Subcutaneous injection of the incretin hormone glucagon-like peptide 1 abolishes postprandial glycemia in NIDDM. Diabetes Care 1994; 17: 1039–44
Todd JF, Wilding JP, Edwards CM, et al. Glucagon-like peptide-1 (GLP-1): a trial of treatment in non-insulin-dependent diabetes mellitus. Eur J Clin Invest 1997; 27: 533–6
Gutniak M, Orskov C, Holst JJ, et al. Antidiabetogenic effect of glucagon-like peptide-1 (7–36)amide in normal subjects and patients with diabetes mellitus. N Engl J Med 1992; 326(20): 1316–22
Dupre J, Behme MT, Hramiak IM, et al. Glucagon-like peptide I reduces postprandial glycemic excursions in IDDM. Diabetes 1995; 44: 626–30
Dupre J, Behme MT, Hramiak IM, et al. Subcutaneous glucagon-like peptide I combined with insulin normalizes postcibal glycemic excursions in IDDM. Diabetes Care 1997; 20: 381–4
Zander M, Taskiran M, Toft-Nielsen MB, et al. Additive glucose-lowering effects of glucagon-like peptide-1 and metformin in type 2 diabetes. Diabetes Care 2001; 24(4): 720–5
Nauck MA, Sauerwald A, Ritzel R, et al. Influence of glucagon-like peptide 1 on fasting glycemia in type 2 diabetic patients treated with insulin after sulfonylurea secondary failure. Diabetes Care 1998; 21(11): 1925–31
Larsen J, Hylleberg B, Ng K, et al. Glucagon-Like Peptide-1 Infusion Must Be Maintained for 24 h/day to Obtain Acceptable Glycemia in Type 2 Diabetic Patients Who Are Poorly Controlled on Sulphonylurea Treatment. Diabetes Care 2001; 24(8): 1416–21
Zander M, Madsbad S, Madsen JL. Effect of 6-week course of glucagen-like peptide 1 on glycemic control, insulin sensitivity, and beta-cell function in type 2 diabetes: a parallel-group study. Lancet 2002 Mar 9; 359(9309): 824–30
Nauck M, Stockmann F, Ebert R, et al. Reduced incretin effect in type 2 (non-insulin-dependent) diabetes. Diabetologia 1986; 29: 46–52
Toft-Nielsen MB, Madsbad S, Holst JJ. Determinants of the effectiveness of glucagon-like peptide-1 in type 2 diabetes. J Clin Endocrinol Metab 2001; 86(8): 3853–60
Toft-Nielsen MB, Damholt MB, Madsbad S, et al. Determinants of the impaired secretion of glucagon-like peptide-1 in type 2 diabetic patients. J Clin Endocrinol Metab 2001; 86(8): 3717–23
Vaag AA, Holst JJ, Volund A, et al. Gut incretin hormones in identical twins discordant for non-insulin-dependent diabetes mellitus (NIDDM)—evidence for decreased glucagon-like peptide 1 secretion during oral glucose ingestion in NIDDM twins. Eur J Endocrinol 1996; 135(4): 425–32
Kieffer TJ, McIntosh CHS, Pederson RA. Degradation of glucose-dependent insulinotropic polypeptide and truncated glucagon-like peptide 1 in vitro and in vivo by dipeptidyl peptidase IV. Endocrinology 1995; 136: 3585–96
Mentlein R, Gallwitz B, Schmidt WE. Dipeptidyl-peptidase IV hydrolyses gastric inhibitory polypeptide, glucagon-like peptide-1 (7–36) amide, peptide histidine methionine and is responsible for their degradation in human serum. Eur J Biochem 1993; 214: 829–35
Mentlein R. Dipeptidyl-peptidase IV (CD26)—role in the inactivation of regulatory peptides. Regul Pept 1999; 85(1): 9–24
De Meester I, Korom S, Van Damme J, et al. CD26, let it cut or cut it down. Immunol Today 1999; 20(8): 367–75
Pauly RP, Rosche F, Wermann M, et al. Investigation of glucose-dependent insulinotropic polypeptide-(l–42) and glucagon-like peptide-l-(7–36) degradation in vitro by dipeptidyl peptidase IV using matrix-assisted laser desorption/ionization-time of flight mass spectrometry: a novel kinetic approach. J Biol Chem 1996; 271(38): 23222–9
Marguet D, Baggio L, Kobayashi T, et al. Enhanced insulin secretion and improved glucose tolerance in mice lacking CD26. Proc Natl Acad Sci U S A 2000; 97(12): 6874–9
Deacon CF, Johnsen AH, Holst JJ. Degradation of glucagon-like peptide-1 by human plasma in vitro yields an N-terminally truncated peptide that is a major endogenous metabolite in vivo. J Clin Endocrinol Metab 1995; 80: 952–7
Wettergren A, Wojdemann M, Holst JJ. The inhibitory effect of glucagon-like peptide-1 (7–36)amide on antral motility is antagonized by its N-terminally truncated primary metabolite GLP-1 (9–36)amide. Peptides 1998; 19(5): 877–82
Deacon CF, Nauck MA, Toft-Nielsen M, et al. Both subcutaneously and intravenously administered glucagon-like peptide 1 are rapidly degraded from the NH2-terminus in type II diabetic patients and in healthy subjects. Diabetes 1995; 44: 1126–31
Hansen L, Deacon CF, Orskov C, et al. Glucagon-like peptide-1-(7-36) amide is transformed to glucagon-like peptide-l-(9–36)amide by dipeptidyl peptidase IV in the capillaries supplying the L cells of the porcine intestine. Endocrinology 1999; 140(11): 5356–63
Ritzel U, Leonhardt U, Ottleben M, et al. A synthetic glucagon-like peptide-1 analog with improved plasma stability. J Endocrinol 1998; 159(1): 93–102
Deacon CF, Knudsen LB, Madsen K, et al. Dipeptidyl peptidase IV resistant analogues of glucagon-like peptide-1 which have extended metabolic stability and improved biological activity. Diabetologia 1998; 41: 271–8
Burcelin R, Dolci W, Thorens B. Long-lasting antidiabetic effect of a dipeptidyl peptidase IV-resistant analog of glucagon-like peptide-1. Metabolism 1999; 48(2): 252–8
Xiao Q, Giguere J, Parisien M, et al. Biological activities of glucagon-like peptide-1 analogues in vitro and in vivo. Biochemistry 2001; 40(9): 2860–9
Siegel EG, Gallwitz B, Scharf G, et al. Biological activity of GLP-1-analogues with N-terminal modifications. Regul Pept 1999; 79(2–3): 93–102
O’Harte FP, Mooney MH, Lawlor A, et al. N-terminally modified glucagon-like peptide-1 (7–36) amide exhibits resistance to enzymatic degradation while maintaining its antihyperglycaemic activity in vivo. Biochim Biophys Acta 2000; 1474(1): 13–22
Knudsen LB, Nielsen PF, Huusfeldt PO, et al. Potent derivatives of glucagon-like peptide-1 with pharmacokinetic properties suitable for once daily administration. J Med Chem 2000; 43(9): 1664–9
Larsen PJ, Fledelius C, Knudsen LB, et al. Systemic administration of the long-acting GLP-1 derivative NN2211 induces lasting and reversible weight loss in both normal and obese rats. Diabetes 2001; 50(11): 2530–9
Eng J, Kleinman WA, Singh L, et al. Isolation and characterization of exendin 4, an exendin 3 analogue from Heloderma suspectum venom. J Biol Chem 1992; 267: 7402–5
Goke R, Fehmann H-C, Linn T, et al. Exendin-4 is a high potency agonist and truncated exendin-(9–39)-amide an antagonist at the glucagon-like peptide 1-(7–36)-amide receptor of insulin-secreting b-cells. J Biol Chem 1993; 268: 19650–5
Thorens B, Porret A, BYhler L, et al. Cloning and functional expression of the human islet GLP-1 receptor: Demonstration that exendin-4 is an agonist and exendin-(9–39) an antagonist of the receptor. Diabetes 1993; 42: 1678–82
Schepp W, Schmidtler J, Riedel T, et al. Exendin-4 and exendin-(9–39)NH2: Agonist and antagonist, respectively, at the rat parietal cell receptor for glucagon like peptide-l-(7–36)NH2. Eur J Pharmacol Mol Pharmacol 1994; 269: 183–91
Fehmann HC, Jiang J, Schweinfurth J, et al. Stable expression of the rat GLP-I receptor in CHO cells: activation and binding characteristics utilizing GLP-I (7–36)-amide, oxyntomodulin, exendin-4, and exendin (9–39). Peptides 1994; 15: 453–6
Edwards CM, Stanley SA, Davis R, et al. Exendin-4 reduces fasting and postprandial glucose and decreases energy intake in healthy volunteers. Am J Physiol Endocrinol Metab 2001; 281(1): E155–61
Young AA, Gedulin BR, Bhavsar S, et al. Glucose-lowering and insulin-sensitizing actions of exendin-4: studies in obese diabetic (ob/ob, db/db) mice, diabetic fatty Zucker rats, and diabetic rhesus monkeys (Macaca mulatta). Diabetes 1999; 48(5): 1026–34
Szayna M, Doyle ME, Betkey JA, et al. Exendin-4 decelerates food intake, weight gain, and fat deposition in Zucker rats. Endocrinology 2000; 141(6): 1936–41
Al-Barazanji KA, Arch JR, Buckingham RE, et al. Central exendin-4 infusion reduces body weight without altering plasma leptin in (fa/fa) Zucker rats. Obes Res 2000; 8(4): 317–23
Greig NH, Holloway HW, De Ore KA, et al. Once daily injection of exendin-4 to diabetic mice achieves long-term beneficial effects on blood glucose concentrations. Diabetologia 1999; 42(1): 45–50
Holst JJ, Deacon CF. Inhibition of the activity of dipeptidyl-peptidase IV as a treatment for type 2 diabetes. Diabetes 1998; 47: 1663–70
Deacon CF, Hughes TE, Holst JJ. Dipeptidyl peptidase IV inhibition potentiates the insulinotropic effect of glucagon-like peptide 1 in the anesthetized pig. Diabetes 1998; 47(5): 764–9
Pauly RP, Demuth HU, Rosche F, et al. Improved glucose tolerance in rats treated with the dipeptidyl peptidase IV (CD26) inhibitor Ile-thiazolidide. Metabolism 1999; 48(3): 385–9
Pederson RA, White HA, Schlenzig D, et al. Improved glucose tolerance in Zucker fatty rats by oral administration of the dipeptidyl peptidase IV inhibitor isoleucine thiazolidide. Diabetes 1998; 47(8): 1253–8
Balkan B, Kwasnik L, Miserendino R, et al. Inhibition of dipeptidyl peptidase IV with NVP-DPP728 increases plasma GLP-1 (7–36 amide) concentrations and improves oral glucose tolerance in obese Zucker rats. Diabetologia 1999; 42(11): 1324–31
Ahren B, Holst JJ, Martensson H, et al. Improved glucose tolerance and insulin secretion by inhibition of dipeptidyl peptidase IV in mice. Eur J Pharmacol 2000; 404(1-2): 239–45
Morimoto C, Schlossman SF. The structure and function of CD26 in the T-cell immune response. Immunol Rev 1998; 161: 55–70
Fleischer B. CD26: a surface protease involved in T-cell activation. Immunol Today 1994; 15(4): 180–4
Tibaduiza EC, Chen C, Beinborn M. A small molecule ligand of the glucagon-like peptide 1 receptor targets its amino-terminal hormone binding domain. J Biol Chem 2001; 276(41): 37787–93
Nauck MA, Holst JJ, Willms B, et al. Glucagon-like peptide 1 (GLP-1) as a new therapeutic approach for type 2-diabetes. Exp Clin Endocrinol Diabetes 1997; 105(4): 187–95
Ranganath LR, Beety JM, Morgan LM, et al. Attenuated GLP-1 secretion in obesity: cause or consequence? Gut 1996; 38: 916–9
Gutniak MK, Larsson H, Sanders SW, et al. GLP-1 tablet in type 2 diabetes in fasting and postprandial conditions. Diabetes Care 1997; 20: 1874–9
Joseph JW, Kalitsky J, St-Pierre S, et al. Oral delivery of glucagon-like peptide-1 in a modified polymer preparation normalizes basal glycemia in diabetic db/db mice. Diabetologia 2000; 43(10): 1319–28
Burcelin R, Rolland E, Dolci W, et al. Encapsulated, genetically engineered cells, secreting glucagon-like peptide-1 for the treatment of non-insulin-dependent diabetes mellitus. Ann N Y Acad Sci 1999; 875: 277–85
Matthews DR, Cull CA, Stratton IM, et al. UKPDS 26: sulphonylurea failure in non-insulin-dependent diabetic patients over six years. UK Prospective Diabetes Study (UKPDS) Group. Diabet Med 1998; 15(4): 297–303
Acknowledgements
DJ Drucker is supported in part by operating grants from the Juvenile Diabetes Research Foundation, the Canadian Diabetes Association, and a Senior Scientist Award from the Canadian Institutes of Health Research. DJ Drucker is also a Consultant to Merck Research Laboratories, Amylin Pharmaceuticals Inc., Conjuchem Inc., and Triad Pharmaceuticals.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Baggio, L.L., Drucker, D.J. Harnessing the Therapeutic Potential of Glucagon-Like Peptide-1. Mol Diag Ther 1, 117–125 (2002). https://doi.org/10.2165/00024677-200201020-00005
Published:
Issue Date:
DOI: https://doi.org/10.2165/00024677-200201020-00005