Abstract
A number of cancer vaccine strategies for the treatment of colorectal cancer have entered clinical trials. Whole tumor cell vaccines have been developed from both patients’ autologous tumor cells as well as established allogeneic tumor cell lines. A vaccine consisting of autologous tumor cells along with bacillus Calmette-Guerin (BCG) has shown a potential clinical benefit in patients with stage II colon cancer. Other approaches using autologous tumor cells have involved transfection of primary tumor cells with cytokine genes. Allogeneic tumor cell vaccines have also been modified to express cytokine genes.
Vectors have been studied extensively as a means of vaccine strategy. One tumor-associated antigen (TAA) that has been extensively studied in viral vector vaccines is carcinoembryonic antigen (CEA). A recombinant vaccinia virus containing the CEA transgene (rV-CEA) has been shown to elicit CEA-specific immune responses in advanced carcinoma patients. However, patients receiving multiple vaccinations had limited increases in CEA-specific responses by the third vaccination. This problem may be overcome by the use of non-replicating poxviruses, which have been shown in clinical trials to be safe and to elicit CEA-specific responses. However, recent clinical studies have shown that the optimal use of poxviruses is to prime with vaccinia, followed by boosts with avipox vectors. A recent randomized clinical trial showed that patients primed with rV-CEA and boosted with avipox-CEA had greater immune responses compared with patients receiving three 1-monthly avipox-CEA vaccinations followed by an rV-CEA vaccination. Furthermore, a statistically significant survival advantage was noted in the prime/boost arm. Ongoing studies are now incorporating the genes for costimulatory molecules along with TAA in these vectors.
Another vaccine strategy involving TAA that is currently in clinical trials for colorectal cancer is the peptide vaccine. Dendritic cells (DCs) are considered to be the most potent antigen-presenting cell, thus providing an attractive modality for cancer vaccines. In addition to using DCs for peptide-based vaccines, a number of other strategies, including transfection with messenger RNA, have produced specific T-cell responses in clinical trials. In addition, several clinical trials using murine anti-idiotype antibodies as vaccines for patients with advanced colorectal cancer have shown both immunologic responses as well as clinical responses.
Similar content being viewed by others
As our understanding of immunology has evolved, new strategies for therapeutic vaccines have been brought forth from the laboratory and are now undergoing clinical evaluation for a wide variety of human carcinomas. Colorectal cancer is the third leading cause of death in both men and women in the US, with approximately 135 000 new cases diagnosed and 57 000 related deaths each year.[1]The 5-year survival rate for patients diagnosed with early localized colon cancer is approximately 90%.[1]However, this rate decreases to 65% when the cancer spreads to the lymph nodes, and to <10% after the development of distant metastasis, despite current treatment strategies including surgery, chemotherapy, and radiation.
The role for adjuvant chemotherapy thus far has been limited to lymph node positive disease with a minor effect on survival.[2]Furthermore, despite newer chemotherapy regimens for metastatic disease, these agents are toxic and have a minor effect on overall survival.[2]The recent data from Saltz et al.[2]using a regimen consisting of irinotecan, fluorouracil, and leucovorin produced only a modest increase in median survival of 15 months for patients with metastatic colon cancer compared with fluorouracil/leucovorin. Furthermore, an increase in toxicity compared with fluorouracil/leucovorin, most notably diarrhea, was observed in these patients. New approaches to the treatment of colorectal cancer are being developed, and a number of molecular-targeted agents are currently being investigated. This article reviews the cancer vaccines currently being used to treat colorectal cancer.
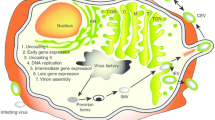
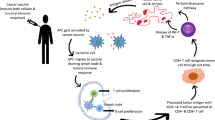
Tumor antigens are proteins expressed by malignant cells that can stimulate immune responses against them. These antigens may represent surface proteins specific to the tumors, or they may be expressed on normal cells, but at much lower quantities than on the tumors. In the 1950s, animal models were established demonstrating that the immune system can specifically prevent the growth of malignant tumors. It was shown that the rejection of these tumors was mediated mainly by tumor-specific cytotoxic T lymphocytes (CTLs). CTLs have receptors that are responsible for the recognition of highly specific peptide fragments of the protein, referred to as epitopes. These epitopes are presented on the surface of antigen-presenting cells (APCs) coupled to the major histocompatibility complex (MHC) molecules (figure 1), also called human leukocyte antigens (HLA) in humans, which are divided into two classes. The class I MHC complex is expressed on most nucleated cells and presents the antigen as short peptides, generally around 8–11 amino acids in length, to CD8+ T cells. The class II MHC molecules are expressed predominantly on specialized APCs and present longer peptides, usually between 11 and 15 amino acids in length, to CD4+ T cells.
T-cell activation requires two signals: signal one, which is antigen specific, and signal two, which is mediated through a costimulatory molecule on the APC that interacts with its ligand on the T-cell receptor (TCR). These costimulatory molecules send signals that help regulate the functional responses of the T cells with which they interact. Certain costimulatory molecules (see section 3.1) such as intercellular adhesion molecule (ICAM)-1 can increase the strength of adhesion between the CTL and APC, further enhancing the activation of the T cell (figure 2). The most potent APCs capable of presenting an antigen to naive T cells are dendritic cells (DCs). In order to initiate a T-cell response, antigenic peptides must be recognized via the TCR of circulating T cells. Tumors often lack MHC molecules and usually lack costimulatory signals. DCs have high levels of expression of both these markers, and therefore have been considered good candidates for vaccine development. DCs are found in most tissues in an immature state where they are able to capture and process antigens efficiently, which can then be presented by both class I and class II MHC molecules to activate CD8+ and CD4+ T cells, respectively.
Another mechanism for downregulation of the immune response, in addition to lack of costimulation, is an antigen-induced block, termed immunologic tolerance. This may result from the interaction of an antigen with a lymphocyte, which, instead of becoming activated, is rendered unresponsive. Tolerance is a fundamental property of the immune system that protects against autoimmunity to self-antigens. The question that arises is how a tumor vaccine can overcome tolerance if the vaccine incorporates a self-antigen expressed on the tumor cell. The ‘danger’ model has theorized that the source of antigen is less important than how it may be presented to the immune system.[3]If a vaccine strategy is developed that elicits a high enough level of immune stimulation, then the antigen can be seen as ‘dangerous’ and immune responses will be stimulated.[4]Some tumor cells may escape being recognized by the immune system by not producing a ‘danger’ signal that adequately reaches a high enough threshold.[5]
A number of vaccination strategies have now been developed based on our current understanding of tumor immunology and how these vaccines can be successfully administered in the clinic. These included:
-
whole tumor vaccines: (i) autologous; and (ii) allogeneic
-
vector-encoded tumor-associated antigens (TAAs): (i) prime and boost strategy; and (ii) costimulatory molecules
-
anti-idiotypes
-
dendritic cell vaccines.
Many of these vaccines have been analyzed in experimental models and have been incorporated into ongoing or completed clinical trials in colorectal cancer. Each of these vaccine types has its advantages and disadvantages, and it may eventually be determined that some of these modalities are most beneficial when used in tandem. However, there are a number of considerations when interpreting the success of various vaccine strategies. With regard to study design, factors such as patient selection play an important role. Patients with extensive metastatic disease who have undergone numerous chemotherapy cycles may not respond to a particular vaccine strategy. However, this same strategy may be successful when treating patients with less disease burden or those who have not been heavily pretreated with drugs that may suppress the immune system. In the adjuvant setting, however, it may take years to determine whether a vaccine strategy is successful. Thus, the role of immunologic assays as intermediate endpoints to assess a response to a vaccine becomes highly significant. One must be cautious that the assay chosen is reliable and that the target is validated as a surrogate marker.
1. Whole Tumor Cell Vaccines
Whole cell vaccines can be separated into two categories: (i)autologous (using a patient’s own tumor cells for vaccination); and (ii) allogeneic (using tumor cells from other patients, usually from established tumor cell lines, for vaccination).
A possible advantage for the use of an autologous whole tumor cell vaccine approach is that tumor antigens specific to the patient undergoing therapy would be present in the vaccine preparation. However, preparing this type of vaccine can be a laborious process. At the time of surgery, an adequate number of tumor cells must be obtained to develop the vaccine and the cells must be prepared in a similar manner for each patient. In addition, to obtain an adequate number of cells, other factors may contribute to the difficulty of preparing the vaccine; these include the percentage of tumor cells and the degree of tumor necrosis. Furthermore, a central laboratory is frequently required to prepare these vaccines from tumors.
1.1 Autologous Vaccines
A number of clinical trials with autologous tumor vaccines have been completed in patients with colorectal cancer (see table I). Intracel Corporation (Frederick, MD, USA) has sponsored studies in patients with stage II and stage III colon cancer given autologous tumor cells mixed with bacillus Calmette-Guerin (BCG) through intradermal vaccinations in an adjuvant setting.[6–8]BCG vaccines have been given to billions of people since 1921 for the prevention of tuberculosis; these vaccines have been administered more than any other vaccine in the world.[9]Indeed, BCG has been used extensively in the immunotherapy of human cancer.[10]In clinical trials in colorectal cancer, patients were randomized to either a control arm or a vaccine-treatment arm after surgical resection of the primary tumor and stratification by disease location and stage. Patients who were randomized to vaccination received 107 irradiated tumor cells with 107 BCG organisms per week for 2 weeks, followed by a boost of 107 irradiated tumor cells in the third week.
The initial studies using this approach were performed at Johns Hopkins Hospital (Baltimore, MD, USA) in the early 1980s.[7]Ninety-eight patients were randomized post-operatively to either surgery alone or surgery with vaccine. No serious adverse effects occurred in the vaccinated patients. All patients developed superficial skin ulcerations at the site of the BCG vaccine. Patients were stratified by tumor stage and as having either rectal or colon cancer. In the colon cancer patients (Dukes B and C) at 6.5 years follow-up, 20 of 24 patients remained alive compared with 12 of 23 patients in the control group. These results were statistically significant (p = 0.02). In addition, there was a statistically significant difference in the number of recurrences in the colon cancer patients who received the vaccine as compared with the control arm (p = 0.03). No significant differences were seen in the rectal cancer patients who received the vaccine as compared with the control group.[7]
The results of this study led to a large phase III trial performed by the Eastern Cooperative Oncology Group (ECOG).[8]In this study, 412 patients with stage II and stage III colon cancer were enrolled to receive the same vaccine regimen as those patients entered in the previously mentioned study.[7]This study was different, however, in that it was conducted at multiple clinical centers and each site was allowed to perform its own manufacturing of the vaccine, based on common specifications provided by Intracel Corporation. At a 7.6-year median follow-up, there were no statistically significant differences in the clinical outcome between the vaccine and the control group. A delayed type hypersensitivity (DTH) test was performed by intradermal injection of the antigen used in the vaccination. The characteristic response of the DTH test occurs over a 24- to 48-hour period after vaccination. This test can be performed after subsequent vaccinations to determine whether a patient continues to respond to vaccination. In a subset analysis, those patients who demonstrated a significant DTH response had a statistically significant improvement in outcome. This ECOG study concluded that not having a common manufacturing laboratory to make the vaccine may have had an impact on the overall results of the study.[8]
A prospective randomized controlled trial was performed in Europe in which 254 colorectal cancer patients were enrolled.[6]This trial differed from an earlier ECOG study in that the investigators used a central facility to prepare the vaccination. Furthermore, patients in the present study received an additional boosting vaccination at 3 months. At 5.3-year median follow-up, there were 40 cancer recurrences in the control group and 25 in the vaccine group. The vaccine showed a statistically significant clinical benefit in the subset of patients with surgically resected stage II colon cancer, but not stage III colon cancer.[6]
Another approach using allogeneic tumor cell vaccines, is the transfection of primary tumor cells with cytokine genes (see table I). A number of investigators have studied the approach of modifying tumor cells by transfecting cytokine genes including interleukin (IL)-7 and granulocyte-macrophage colony-stimulating factor (GM-CSF).[11,23–28]It was postulated that GM-CSF activates DCs, leading to enhanced antigen presentation of the tumor-derived antigens. In addition, the interleukin leads to direct stimulation and proliferation of immunologic antitumoral effector cells.[27]Fourteen primary cell cultures were established from 45 patients with malignant melanoma. These cell cultures were then transfected with the gene encoding for human IL-7. This resulted in the production of biologically active IL-7 production by the tumor cells. No differences in the phenotype were observed between the IL-7 transfected cells compared with those cells that were not transfected. The expression of MHC class I and II, ICAM-1, as well as melanoma-associated antigen was unchanged following transfection. The IL-7 transfected cell cultures possessed a higher sensitivity to immunologic effector cells compared with nontransfected cells.[28]These results led to a recent study by Wittig et al.,[11]in which ten patients with progressive metastatic carcinoma, including those with colorectal cancer, were vaccinated using this approach. Autologous tumor cells were transfected with what the authors termed a minimalistic, immunogenically defined, gene expression construct (MIDGE) for overexpression of IL-7 and GM-CSF. The transfection of the cytokines was performed ex vivo using a ballistic approach that launches seven particle carrier membranes simultaneously, in such a way as to evenly distribute ballistic microparticles for transfection in to more than 1.5 × 107 target cells in one shot, combined with a magnetic separation of transfected cells. All ten patients enrolled had progressive disease prior to enrollment. Patients received four subcutaneous injections of at least 1 × 106 of their cytokine modified tumor cells. The cytotoxic effects of treatment on patient-derived peripheral blood lymphocytes (PBLs) were monitored during the treatment, using autologous tumor cells as targets. PBLs were obtained from patients before, during, and after treatment, and were found to increase significantly during treatment (p = 0.01). One complete, one partial, and one mixed response with progression of abdominal metastases and regression of lung metastases were observed.[11]
1.2 Allogeneic Vaccines
Allogeneic whole tumor cell vaccines usually consist of one or more tumor cell lines and are relatively easy to prepare compared with autologous vaccines. This vaccine approach may also contain several tumor-specific antigens (TSAs) and/or TAAs. Moreover, the cell lines used in the preparations can be infected with vectors that express cytokine genes (such as GM-CSF)[29]or costimulatory molecule genes (such as B7-1)[30]to enhance the immunogenicity of the tumor cell. Allogeneic whole tumor cell vaccines modified to secrete GM-CSF have been used in a phase I trial in patients with pancreatic cancer.[31]Evidence of prolonged disease-free survival has been seen in some patients for at least 25 months after diagnosis. Increases in immune responses were also observed as measured by DTH.[31]Clinical studies are ongoing in patients with gastrointestinal carcinomas using either peptides or viral vector vaccines using the target antigens p53,[32]MAGE,[33]or the ras oncogene.[34,35]
An allogeneic tumor cell vaccine termed CancerVax® 1 was developed at the John Wayne Cancer Institute (Santa Monica, CA, USA) [see table I]. This is a polyvalent vaccine consisting of three live human melanoma cell lines chosen for their wide range of TAAs and MHC antigens. The immune response to CancerVax® has been shown to cross-react with nonvaccine tumor cells expressing some of the same immunogenic TAAs, such as gangliosides (GD2, GM2, GD3, and GM3), glycoproteins (fetal antigen, TA90), and/or proteins (MAGE-1, MAGE-3).[36]The use of this vaccine in melanoma patients has produced both immunologic as well as clinical responses.[37–42]IgM responses to a specific TAA, TA90, have been shown to predict survival in patients receiving CancerVax® adjuvant immunotherapy for stage III and stage IV melanoma.[43–45]Furthermore, the TA90 antigen has been shown to be expressed on a wide variety of solid tumors, including colorectal cancer. In a recently published study, 27 patients with stage IV colorectal adenocarcinoma were treated with CancerVax® (at weeks 0, 2, 4, 6, 8, and every fourth week successively for 1 year) co-administered with BCG (for the first 2 weeks of vaccine treatment). There was a significant (p = 0.0001) increase in anti-TA90 IgG and IgM titers and in DTH response to vaccine cells. The median overall survival was 21.9 months for the entire group. The authors correlated improved survival to immune responses.[12]
A number of clinical trials using whole cell vaccines are now actively accruing patients (see table II). Intracel Corporation is sponsoring a multicenter phase I/II study of adjuvant autologous tumor cell vaccination in patients with completely resected stage II or III colon cancer. After total surgical resection, patients receive autologous tumor cell vaccine intradermally once weekly for three vaccinations, with the first two vaccinations also containing BCG. Patients with stage III disease receive standard adjuvant chemotherapy with fluorouracil and leucovorin following vaccination. All patients receive a fourth vaccination about 6 months after surgical resection. Another phase II study is assessing autologous tumor cells (or, if unavailable, allogeneic) incubated with interferon, then irradiated and reinfused by dorsal pedal cannulation. Cyclophosphamide is given once 3 days prior to infusion, and GM-CSF daily for 9 days after infusion.
A disadvantage in the use of allogeneic vaccines prepared from whole tumor cells is that they do not constitutively express costimulatory molecules. Moreover, it is possible that since allogeneic cells are used, alloimmunity to non-self components may develop. However, this was not the case in early clinical trials, which include studies using oncolysates (i.e. tumor cell preparations that had been infected with vaccinia virus and then lysed in an effort to enhance their immunogenicity).[46]
Thus, in summary, the major advantage of using whole tumor cell vaccines is that several TSAs or TAAs, some of which have yet to be defined, may be present in the vaccine preparation. However, this approach has major disadvantages as well. The actual amount of any TAA or TSA in the vaccine composition may be diluted by normal cellular components of the tumor. The vast majority of solid tumors, including colorectal cancer, do not express costimulatory molecules, which are responsible for the activation of naive T cells to levels capable of inducing therapeutic responses. Finally, it is unclear which immunogenic proteins or epitopes are present in the vaccine, thus making it difficult to measure immune responses and to amplify those specific responses.
2. Tumor-Associated Antigen (TAA) Strategies
2.1 Early Strategies
In the early 1970s, using colony inhibition assays, Hellstrom et al.[47]demonstrated that lymphocytes from colorectal cancer patients would prevent colony formation by autologous as well as homologous tumor cells. This work suggested the presence of cross-reactive TAAs in colorectal cancer as well as the presence of individual specific TSA. Elias et al.[48]further demonstrated specific cell-mediated immunity in patients with Dukes B and C colon cancer as measured by an autologous leukocyte migration inhibition assay (MIA). The principle behind this assay is that the migration of peripheral human blood leukocytes is inhibited when leukocytes are exposed in vitro to an antigen to which they have previously been sensitized. Immunity was observed prior to surgery and in the immediate postoperative period, but disappeared shortly afterwards. Furthermore, immunity could not be demonstrated by MIA against colon cancer TAA, when lymphocytes were tested from patients with Dukes D colon cancer, patients with other cancers, or from healthy volunteers.[48]This suggested that these antigens were specific for the tumor; however, more advanced disease may suppress the immune responses.
A phase I study by Hollinshead et al.[49]explored the use of TAA therapy in patients with adenocarcinoma of the colon. The TAA was derived from the patient’s colon cancer, which was obtained post-operatively. Cell membranes were separated from the tumor, and soluble membrane proteins were removed by Sephadex B-200 chromatography. Semipurified TAAs were identified by in vitro and in vivo testing in colon cancers and controls for cell-mediated immunoreactivities, and colon TAAs were identified in fetal intestine cell membranes and on tumor cell membranes. Using discontinuous, gradient gel electrophoresis, both colon TAA and carcinoembryonic antigen (CEA) were separated and eluted and cross-compared, with TAA shown to be separate from CEA. The TAA consisted of two stable polypeptides with approximate molecular weights of 72kD and 88kD, respectively, as compared with the 180kD molecular weight of CEA. For the phase I trial, TAAs were prepared from the tumors of 70 selected hepatitis-free donors; they were tested for standard potency after sterility, and general safety tests were performed. The final product was dispersed in 200, 300, and 500μg TAA protein concentrations per 0.2mL. Vaccines were prepared by mixing TAA 0.2mL with complete Freund’s adjuvant (CFA) 0.2mL.
Twenty-two patients received vaccinations with follow-up ranging from 3 months to 3 years (median 21 months). Seven patients had Dukes B2, seven had Dukes C, and eight had Dukes D stage colon cancer. All patients underwent surgical resection, with six of eight patients with Dukes D clinically disease free at the time of vaccination. Each patient received three 1-monthly vaccinations. Two patients received 200μg doses of TAA, two received 500μg doses of TAA, and 18 received 300μg doses. All patients developed skin ulcers at the vaccination site, all of which healed and formed a scar. Two patients developed fever and chills during the first day post-vaccination, which resolved with acetaminophen. There were no clinical or biochemical manifestations of any type of systemic toxicity. Patients were tested for DTH reactions to skin tests with 50μg protein TAA alone. A reaction of >5mm in duration was considered a positive test. One patient had a negative response to serial skin testing; three patients showed a positive response, but this decreased during months 6, 9, and 10, respectively. The remaining patients showed steady positive responses; in general, the greatest responses were noted between 5 and 6 months post-therapy. MIA was also performed in patients with Dukes B and C stages. A patient receiving the 200μg protein TAA did not show as pronounced a reaction as patients receiving 300μg or 500μg doses. Furthermore, at 4–5 months post-vaccination, there was a switch from ± to positive reactions in all patients. This concurred with the DTH results, which demonstrated that strongest responses occurred approximately 5 months post-vaccination. Although this was a small phase I trial indicating the safety of the vaccine, at the median follow-up of 21 months, 82% of the patients were still alive, and 59% of the patients were without evidence of disease.
2.2 Vector-Encoded TAA
Vectors have been studied extensively as a means of vaccine delivery and a number of review articles have been published describing the use of these vectors for cancer vaccines.[50–55]Strategies incorporating the use of both viral and bacterial vectors are now in use in the clinic. Each of these vectors has its own advantages and disadvantages. The advantages to using a vector-based vaccine are: (i) the entire tumor antigen gene or parts of that gene can be inserted; (ii) multiple genes (including genes for costimulatory molecules and cytokines) can be inserted into some types of vectors; (iii) the relative cost of this type of production is low compared with the preparation and purification of proteins or whole tumor cell vaccines; and (iv) many vectors have the ability to infect ‘professional’ APC so that the antigens they express can be processed.
Viral vectors including poxvirus (vaccinia and avipox) and adenovirus have been extensively used as delivery vehicles for TAA vaccines. Vaccinia virus, which was derived from a benign cutaneous disease in cows, has been administered to more than 1 billion people and is responsible for the worldwide eradication of smallpox.[56]To date, as many as seven transgenes have been expressed in one vaccinia virus vector. Another major advantage is that proteins expressed in vaccinia virus tend to be more immunogenic than the native protein, which is most likely to be attributable to the inflammatory responses triggered against highly immunogenic vaccinia virus proteins. Other advantages of poxviruses, such as vaccinia virus, modified vaccinia Ankera (MVA) and avipox viruses include: (i) a wide host range; (ii) stable recombinants; (iii) accurate replication; and (iv) efficient post-translational processing of the inserted gene.
Adenovirus as a vector for the development of recombinant vaccines is attractive because its viral genome can be altered to accept foreign genes that are stably integrated. To improve the quality of recombinant adenovectors, endogenous viral DNA sequences are typically deleted from replication-competent regions, which results in an attenuated form of the virus. Recombinant adenoviruses have been widely used in gene therapy protocols, and a number of vaccine protocols for the induction of immune responses have already been carried out.[57–60]
One TAA that has been extensively studied in viral vector vaccines is CEA, a 180 000-glycoprotein member of the immunoglobulin super-gene family. Several functions have been attributed to CEA, including homotypic and heterotypic intercellular adhesion. It has also been reported that CEA can cooperate in cellular transformation with several proto-oncogenes, such as BCL2 and c-Myc. CEA is overexpressed on >90% of colorectal cancers and other gastrointestinal tumors, as well as other tumors. A disadvantage to using CEA as a target in immunologic-based therapies is that CEA is a normal protein expressed in the body; thus it is likely that tolerance will exist to this protein. Vaccination with a live recombinant vaccinia virus can help to overcome the problem of tolerance. It allows for the expression of foreign antigens encoded by a transgene directly in various cells of the host, including professional APCs. This method of vaccination enables antigen processing and presentation of antigenic peptides along with host histocompatibility antigens and other necessary co-factors found on the APCs. One of the main advantages of using recombinant vaccinia viruses to develop cancer vaccines, as demonstrated by numerous investigators, is that when a gene for a protein is inserted into recombinant vaccinia and used as an immunogen, the recombinant protein is much more immunogenic than the use of that protein with adjuvant.[61–63]This concept was exemplified by Kass et al.,[62]who showed that two injections of CEA protein in adjuvant generated little, if any, immune response to CEA in a CEA transgenic (CEA-Tg) mouse. This would be expected because the host is seeing CEA as a ‘self’ antigen. However, when the recombinant vaccinia virus containing the CEA transgene (designated rV-CEA) is administered one or two times, a strong CEA-specific T-cell response is elicited.[62]The likely reason for this is that a strong inflammatory response is generated by the host against vaccinia proteins. In turn, this inflammatory process apparently leads to an environment of cytokine production and T-cell proliferation that may further amplify the immune response to the transgene antigen. This process favors induction of both cell-mediated and humoral responses to the transgene antigen. Because vaccinia actively replicates in the host, it can present high levels of transgene antigen to the immune system over a period of approximately 1 week, substantially increasing the potential for immune stimulation. The host-immune response to the vaccinia vector then eliminates the virus.
Several clinical trials have demonstrated the immunogenicity of CEA. Tsang et al.[64]and Cole et al.[65]demonstrated that administration of rV-CEA to advanced carcinoma patients can lead to the induction of CEA-specific immune responses.[64,65]The CTL lines generated were shown to be capable of lysing CEA peptide-pulsed targets and CEA-expressing tumor cells. These studies also demonstrated that as long as ≥107 plaque-forming units (pfu) of rV-CEA were administered, a good ‘take’ (erythema and pustule formation) was observed in all patients who had previously received a childhood smallpox vaccination. These studies also demonstrated the safety of administering a live recombinant vector in patients with advanced cancer. They also showed that while rV-CEA could be administered once, and at most twice, by the third vaccination there was little or no ‘take’; these findings correlated with the lack of increases in CEA-specific responses by the third vaccination.[64,65]Furthermore, no significant antineoplastic effect was observed. Possible reasons for the lack of clinical efficacy in these trials were: (i) the prior exposure to the vaccinia virus in all patients treated, which led to the development of an anti-vaccinia immune response; (ii)the advanced state of the tumors in patients; and (iii) the potentially decreased immune status of patients attributable to prior chemotherapy regimens.
To overcome this problem, non-replicating poxviruses were examined in clinical trials. The poxvirus family contains MVA, a derivative of vaccinia virus.[50]This is a virus that has been passaged in chick embryo fibroblasts over 350 times to decrease the virulence of the virus, and thus has the theoretical advantage from a patient safety standpoint of being able to infect mammalian cells but not to replicate in them. Other replication-defective members of the poxvirus family are the avipox vectors (fowlpox and canarypox/ALVAC).[51]These avipox vectors infect human cells and express their transgenes for 2–3 weeks before undergoing cell death. They are incapable of reinfecting cells. Marshall et al.[13]and Zhu et al.[66]designed a phase I study (n = 18) to define the safety of avipox CEA recombinant in patients with advanced CEA-expressing carcinoma. This study also constituted the first trial of any avipox recombinant vaccine in cancer patients. Safety was demonstrated, as was the generation of statistically significant increases in CEA-specific CTL precursors from peripheral blood mononuclear cells (PBMC) from seven of nine HLA-A2-positive patients after vaccination.[13,66]However, preclinical and recent clinical[14]studies have shown that optimal use of recombinant vaccinia viruses may be to prime the immune response, followed by boost vaccinations with other vectors (such as replication-defective avipox vectors), peptides or proteins.
3. Prime and Boost
Priming with one type of immunogen and boosting with another may be advantageous because some of the most effective methods of vaccination, such as the use of recombinant vaccinia virus or adenoviruses, can be used only for a limited number of times because of host anti-vector responses. Numerous preclinical studies have demonstrated the advantages of diversified prime and boost protocols.[67–71]In an effort to determine which heterologous prime and boost regimen to use, a small randomized trial was conducted to compare the rV-CEA as the initial priming vaccination followed by boosting with avipox-CEA (VAAA) with the three vaccinations with avipox-CEA first, followed by rV-CEA (AAAV).[14]In each group, patients were evaluated for immunologic responses using the enzyme-linked immunosorbent spot (ELISPOT) assay. The ELISPOT assay is relatively sensitive and quantitative. By measuring cytokine release on a single-cell basis, the assay can detect a peptide-specific T-cell response against specific HLA class I binding peptides.[72]This study showed that the immune responses seen in the VAAA arm were much better than those in the AAAV arm. Furthermore, continued follow-up of these patients revealed that although there were only nine patients in each arm, five of nine patients (at the time of a recent presentation) were alive on the VAAA arm (2-year survival estimate 67 ± 19%), whereas in the AAAV arm, zero of nine patients were alive (2-year survival estimate 0 ± 0%).[73]This survival difference was related to the immune response; thus, those patients who had at least a 2.5-fold increase in their CEA-specific T cells lived longer (p = 0.03).
3.1 Costimulatory Molecules
Destruction of immunologic targets (such as tumors) requires T-cell lymphocyte recognition (via the TCR) of antigenic peptides presented in the context of MHC molecules on APCs. Costimulatory molecules are critical in the generation of potent T-cell responses. The initiation of an immune response requires at least two signals for the activation of naive T cells by APCs. The first signal is antigen specific, delivered through the TCR via the peptide/MHC, and causes the T cell to enter the cell cycle. The second ‘costimulatory’ signal is required for cytokine production and proliferation. The most extensively studied pathway of costimulation is that involving the interaction of the costimulatory molecule B7-1 (CD80) expressed on APC with cd28 and ctla4 on the T cell.[74–77]A second B7 family member, B7-2 (CD86), has also been identified that interacts with the same T-cell ligands as B7-1. However, B7-2 is upregulated earlier in APC stimulation, and then decreases as B7-1 levels increase. During DC maturation, another important molecule is strongly upregulated, together with costimulatory molecules such as B7-1 and B7-2. This molecule known as leukocyte function-associated antigen (LFA)-3 (CD83) is one of the best-known maturation markers for human DCs. The fact that CD83 is strongly upregulated together with costimulatory molecules such as B7-1and B7-2 during DC maturation suggests that it plays an important role in the induction of immune responses. One mechanism proposed for the ability of tumor cells to evade destruction by the immune system is their failure to express adequate levels of costimulatory molecules, resulting in a failure to induce T-cell responses.[78–81]A corollary of this hypothesis is that the introduction of proper costimulatory molecules into tumors that express TAAs should enhance their ability to elicit specific anti-tumor immune responses. Several studies have demonstrated that transfected tumor cells expressing B7-1 induce potent responses against both modified and unmodified tumor cells.[82,83]B7-1-transfected tumors either failed to grow or, after initial growth, regressed. Furthermore, the immune response induced by B7-1-positive tumors protected animals from re-challenge with untransfected tumor.
The proper engagement of the TCR and costimulatory receptor requires the expression of both antigen and costimulatory molecules, respectively, in the same cell. Therefore, co-expression of costimulatory molecules using a single recombinant vector presents the potential for cooperation among these proteins to enhance T-cell activation.
In a recently completed clinical trial by von Mehren et al.[16]39 patients with CEA-expressing cancers were treated with ALVAC-CEA/B7-1 alone and 30 patients received ALVAC-CEA/B7-1 and GM-CSF (see table I[16,84]). Patients received 4.5 × 108 pfu intradermally every other week, for a total of four injections. The vaccine was again found to be safe, with the major toxicity limited to local erythema and swelling at the vaccine sites. Disease stabilization was seen in 26% of the patients who received the vaccine alone, and in 37% of the patients who received the vaccine in combination with GM-CSF. Increases in the T-cell precursor frequency to a CEA peptide were seen in 10 of 12 patients treated with the vaccine alone and T-cell precursor frequencies as high as 1 in 13 000 were documented.[16]No change in the T-cell precursor frequency recognizing an unrelated flu matrix peptide was documented.
A number of additional costimulatory molecules on APCs have been identified; these include ICAM-1 and LFA-3, whose ligands are LFA-1 and cd2, respectively, on the surface of T cells.[85]Both ICAM-1 and LFA-3 are also capable of conferring similar levels of costimulation of T cells against tumor cells in mouse models.[86,87]Multigene constructs using poxviral vectors (avipox and vaccinia) have been generated. These vectors contain a triad of costimulatory molecule transgenes consisting of B7-1, ICAM-1, and LFA-3, and have been given the designation TRICOM, i.e. rV-TRICOM and avipox-TRICOM. Preclinical studies using TRICOM constructs have shown them to be superior to those constructs that contain one or two of the costimulatory molecules.[69,88,89]
An ongoing phase I clinical trial at Georgetown University (Washington, DC, USA) is evaluating the safety of CEA-TRICOM vectors (see table II). To date, 51 patients have been accrued, completing all six dose-escalation cohorts as well as a seventh cohort with GM-CSF. Only mild treatment-related toxicity has been observed to date. Evidence of clinical activity (resolution of a lung tumor) has been observed in at least one patient treated with only two injections of avipox-CEA/TRICOM. These studies thus indicate the safety profile of the TRICOM vectors.[90,91]
In another phase I trial that is currently accruing, patients with locally advanced or metastatic CEA-expressing cancer are given a pox-vector-based vaccine with CEA/TRICOM genes with GM-CSF or avipox GM-CSF. A third trial evaluating an avipox-CEA based vaccine also contains a T-cell costimulatory molecule (B7-1). In this Aventis-sponsored trial, patients with metastatic colorectal cancer are randomized into three arms with irinotecan alone, irinotecan with vaccine, or the combination of irinotecan with vaccine and tetanus toxoid (see table II).
3.2 Peptides
Peptides are characterized by their ability to induce an immune response by interacting with the appropriate class of the MHC molecule on the APC surface. Peptides of approximately 8–11 amino acids in length, if they possess the appropriate binding motifs, will bind to MHC class I molecules. These peptide-MHC complexes will interact with the TCR to activate CD8+ T cells. These CD8+ T cells, termed cytotoxic T lymphocytes, are usually responsible for lytic destruction of tumors. Peptides of approximately 11–15 amino acids in length, if they contain the appropriate binding motifs, will bind to MHC class II molecules on the surface of T cells. This will lead to the activation of CD4+ or ‘helper’ T cells that produce cytokines and help to promote activation of CD8+ T cells. These ‘MHC-restricted’ responses are thus effective only if the appropriate MHC allele is present in a patient.
The most studied MHC restriction element in the human population is the MHC class I allele, known as HLA-A2, which is found in approximately 50% of all Caucasians. Numerous peptide-binding motifs to HLA-A2 molecules have been identified, and clinical trials are being carried out in which a given cohort of individuals possess the HLA-A2 allele; thus T-cell responses to a particular defined peptide can be quantified. Preclinical studies have shown that both CD8 and CD4+ T cells are usually activated for a vigorous antitumor effect.[92–94]However, studies do exist in which a CD8+ T-cell response alone or a CD4+ T-cell response alone can provide an antitumor effect.[17,95,96]Oligopeptides that contain both class I and class II epitopes can also be used. In a unique set of circumstances, an oligopeptide of the repeat sequences of the mucin (MUC)-1 has been shown to cross-link TCRs in an MHC-unrestricted manner to activate T cells.[97]
This approach is currently being evaluated in a phase I/II study of a ras peptide cancer vaccine with or without IL-2 in HLA-A2-1-positive patients with locally advanced or metastatic colorectal cancer (see table II). Patients are assigned to one of two treatment groups according to extent of disease. Patients with prior locally advanced disease are assigned to treatment group A, while those with metastatic disease are assigned to treatment group B. In group A, patients are vaccinated against influenza on day -6. Patients undergo collection of PBMC on day -4. The PBMC are cultured with sargramostim (GM-CSF) and IL-4 for 5 days and CD40 ligand for 24 hours to differentiate them into DCs and then pulsed for 2 hours with the appropriate peptide (ras) to form a vaccine. Patients receive peptide-pulsed DC vaccine intravenously (IV) over 5 minutes on days 1, 15, 29, 43, and 57. In group B, patients undergo collection of PBMC and receive vaccination as in group A. Patients also receive IL-2 subcutaneously on days 2–6 and 9–13. Treatment repeats every 2 weeks for up to five courses in the absence of disease progression or unacceptable toxicity. Patients are followed up on days 75, 90, 120, and 365.
Using peptides as immunogens has many advantages, including: (i) whole proteins may contain parts of the molecule that are shared with normal cellular proteins, and the use of peptides minimizes the potential for induction of autoimmunity; (ii) preparation is relatively easy and affordable; (iii) because the immunogen is extremely well defined, the immune response can be analyzed in several ways and quantitated; (iv) tetramers, which are molecules that contain specific peptides bound to MHC components, can be used to bind to and isolate antigen-specific T cells induced and amplified in the host; (v) tumors can be stripped of peptide-MHC complexes, and those peptides displayed on the surface of tumors can be identified; and (vi) peptides can be modified to be more immunogenic in the generation of peptide agonists.
3.3 Agonist Peptides
Formation of peptide agonists involves modifying the amino acids of the peptide that bind to either the MHC on the APCs or the TCR. More vigorous MHC binding (i.e. higher affinity for the MHC molecule) often leads to the generation of a more vigorous T-cell response. The advantage of using agonist epitopes has now been demonstrated in clinical trials. An agonist peptide epitope to CEA has been shown to have clinical activity in patients with CEA-expressing tumors.[98]In a recent trial by Fong et al.,[18]patients with CEA-expressing tumors received two 1-monthly vaccinations with DCs loaded with the CEA agonist peptide; 2 of 12 patients experienced complete responses, one patient had a mixed response, and two had stable disease. Clinical response in this trial correlated with CEA-specific T-cell responses.
The agonist CEA epitope is being studied in a phase II randomized trial in HLA-A2-positive patients with CEA-producing adenocarcinomas of gastrointestinal tract origin. Patients are randomized to one of two treatment arms. In arm I, patients receive CEA peptide (CAP 1-6D) emulsified in Montanide ISA-51 adjuvant subcutaneously on day 1. In arm II, patients receive CAP 1-6D dissolved in sargramostim (GM-CSF) intradermally on day 1. Treatment repeats in both arms every 3 weeks for six courses in the absence of disease progression or unacceptable toxicity. Patients are followed up at 3 weeks and then as necessary.
The specificity that a peptide possesses can also be disadvantageous to its use as a cancer vaccine. It is possible that the peptide identified has a dominant CTL epitope, which may induce a CTL response. However, this response may be short-lived because of the lack of ‘help’ provided by helper peptides not present in the vaccine. Furthermore, the use of a peptide vaccine is limited, because patients who do not have that specific allele (e.g. only 50% of the population has the HLA-A2 allele for an HLA-A2-reactive peptide) would not be eligible to receive the vaccine. Combinations of antigenic peptides with reactivities for multiple HLA alleles would circumvent this limitation. Clinical experience with the use of peptides as cancer vaccines is now emerging. Some peptides under study include human papillomavirus (HPV),[99]ras,[92,93]HER-2/neu,[94]MAGE,[100]MART-1, tyrosinase,[101]gp100,[102,103]CEA,[17]MUC-1,[96]PSMA,[104,105]among others.
3.4 Anti-Idiotypes
The concept of vaccinating with anti-idiotypic antibodies is based on the immune network approach described by Jerne.[106]According to this hypothesis, the variable antigen-binding regions of antibodies (Ab1) contain idiotypic determinants that are immunogenic and induce the formation of so-called anti-idiotypic antibodies (Ab2). Some of these Ab2 (‘internal-image’) antibodies are able to functionally mimic the 3-dimensional structure of the original antigen. Thus, selective vaccination with Ab2 could induce a specific immune reaction directed against the original antigen.[107–111]Several small clinical studies using murine Ab2 for the treatment of patients with advanced colorectal carcinoma have demonstrated the induction of antitumoral humoral and cellular immune responses leading to improved clinical responses and tumor regression.[109,112–115]Other studies (discussed in this section) have evaluated anti-idiotype antibodies that mimic TAAs on colorectal cancer cells (see table I).
An anti-idiotypic antibody was generated against murine 17-1A antibody. Six patients with colorectal cancer who underwent surgery were vaccinated with this human anti-idiotype antibody that mimics GA773-2 antigen.[19]All of the patients developed specific T-cell responses against GA733-2, and five of six developed specific IgG antibody response against GA733. Other investigators designed a similar trial, using a rat anti-idiotype antibody generated to the 17-1A antibody.[116]Nine colorectal cancer patients were evaluated in this study following vaccination with aluminum hydroxide precipitated 17-1A. Although four of the nine patients developed a DTH response, no specific antibodies were detected post-vaccination.
The human monoclonal antibody 105AD7 mimics the gp72 antigen that is expressed in 80% of colorectal cancer cells, and can induce a DTH reaction against human tumor cells. In a phase I study with 13 colorectal cancer patients, 105AD7 was administered intramuscularly, and increased levels of IL-2 and a lymphocyte-proliferative response were observed after stimulation in vitro by gp72 antigen-positive cells. When these results were compared with historical controls, the authors suggested that there was a clinical benefit seen with this vaccine.[20]Small trials have demonstrated the ability of 105AD7 to induce significant infiltration of CD4+ cells and natural killer (NK) cells into tumors in vaccinated patients,[117]a significant increase in median CD25+ lymphocytes within the tumor[118]and a significant increase in apoptosis in tumor cells.[119]However, in order to evaluate these promising results more definitively, a prospective, randomized, double-blind, placebo-controlled survival study in patients with advanced colorectal cancer was performed.[21]Patients (n = 162) were randomized to receive three treatments with either 105AD7 or placebo at the time of enrollment, and at 6 and 12 weeks. Study groups were comparable in terms of patient demographics and time from diagnosis of advanced colorectal cancer (277.1 vs 278.6 days). Patient demographics, time of diagnosis of advanced colorectal cancer, and baseline disease were similar, with 50% of patients having malignancy in at least two anatomic sites. Compliance with treatment was poor, with only 50% of the patients receiving three planned vaccinations. Median survival from randomization date was 124 and 184 days in 105AD7 and placebo arms, respectively (p = 0.38), and 456 and 486 days from the date of diagnosis of advanced disease (p = 0.82). In this trial, 105AD7 vaccination did not demonstrate any improvement in survival in patients with advanced colorectal cancer. Although the reason for this was not entirely clear, according to the authors, the high tumor burden and the lack of compliance may have played a role in the poor results.[21]
CeaVac® (Titan Pharmaceuticals, San Francisco, CA, USA) is an anti-idiotype murine monoclonal antibody that mimics a highly tumor-restricted CEA epitope.[22]Polyclonal antibody responses were demonstrated in 17 of 23 patients with advanced colorectal cancer and, in 13 of these patients, anti-CEA responses were detected. Five patients had specific T-cell responses to CEA. None of these patients had objective clinical responses, but overall, median survival for 23 evaluable patients was 11.3 months, with a 44% 1-year survival. Toxicity was limited to a local reaction at the vaccine site with minimal pain incurred.
In a colorectal cancer trial using CeaVac® post-resection, 32 patients were randomized to treatment with aluminum hydroxide-precipitated CeaVac® 2mg intracutaneously or CeaVac® 2mg mixed with QS-21 adjuvant 100μg subcutaneously every other week, then monthly until disease recurrence. Patients participating in this trial had differing stages of disease.[120]Four patients with Dukes stage B2, 11 with Dukes stage C, and eight with Dukes stage D had their tumors completely resected. Nine patients with Dukes stage D carcinoma had their tumors incompletely resected; positive margins post-operatively. Fourteen patients underwent chemotherapy with fluorouracil given concomitantly with CeaVac®. Ten patients relapsed or had progressive disease at time points ranging from 6–30 months. Two patients died at 14 and 20 months, respectively. All 32 patients demonstrated idiotype-specific T-cell responses, of which 75% were CEA specific. These T-cell responses were measured by proliferation of patients’ PBMC in response to CEA. The concomitant use of chemotherapy did not impair this immune response.[120,121]Even though this trial was not designed to examine survival, the authors noted that several of the high risk patients appeared to do better than expected as measured by historical controls. Although this strategy of anti-idiotype vaccines for colorectal cancer may provide patients with some clinical benefit, larger phase III trials are necessary to confirm the clinical benefit of such an approach in the treatment of colorectal cancer.
A number of ongoing trials are currently accruing patients using the approach of anti-idiotype vaccines (table II). One multicenter phase II trial will study the efficacy of adjuvant monoclonal antibody 3H1 anti-idiotype vaccine and monoclonal antibody 11D10 anti-idiotype vaccine in patients with minimal metastatic colorectal cancer after complete hepatic resection. Beginning 6–12 weeks after curative hepatic resection, patients receive monoclonal antibody 3H1 anti-idiotype vaccine and monoclonal antibody 11D10 anti-idiotype vaccine intracutaneously at separate sites on days 1, 15, 29, and 45, then subcutaneously monthly for 2 years beginning on day 73, and then every other month for 3 years.
3.5 Dendritic Cell Vaccines
Antigen presentation is a crucial step in the initiation of an effective immune response that requires the presentation of antigens to sensitize naive T cells and to restimulate primed T cells. The DC is considered the most potent APC, and therefore, one of the most attractive means of vaccination.[122,123]DCs are capable of activating naive CD4+ and CD8+ T lymphocytes by antigen presentation through an MHC-restricted manner. DCs are found in most tissues where they exist in an immature state; in this state they are unable to stimulate T cells but possess an exceptional ability to capture and process antigens. These captured antigens can be presented efficiently by both class I and class II MHC molecules. Antigen capture acts as a signal for the cell to mature and mobilize to regional lymph nodes. These cells undergo extensive transformation, in which antigen capturing decreases and T-cell stimulatory functions increase. The unique capacity of these ‘mature’ DCs to activate T cells is probably related to the presence of an exceptionally high number of MHC, and costimulatory and adhesion molecules.
A number of different immunologic strategies using DCs have been investigated.[123–128]DC vaccines can be employed by: (i) loading with a peptide, protein or anti-idiotype antibody; (ii) infecting with a viral vector; or (iii) loading with apoptotic bodies from tumor cells. The major disadvantage of this strategy is the great cost and effort involved. One must obtain large amounts of peripheral PBMC from patients via leukapheresis. The PBMC must then be cultured for several days in the presence of cytokines such as GM-CSF, IL-4 and/or tumor necrosis factor (TNF)-α, and then reinfused into the patient. This must be done for each patient.
However, DC biology is a rapidly growing field with much promise. In clinical trials, DCs loaded with anti-idiotype antibodies have proved quite successful, resulting in clinical remissions in patients with B-cell lymphoma.[124,125]Other clinical studies using peptide-loaded DCs are under way in a range of human malignancies.[17,105,123,126–128]Generally, DC-based vaccinations have been found to be safe, and can induce antigen-specific T-cell responses and remissions, at least in subsets of patients with advanced disease. A particularly intriguing strategy involves vaccination with DCs that have been ‘fused’ with the host’s own tumor cells, resulting in a DC-tumor cell hybrid vaccine. Significant antitumor responses were observed in both preclinical animal studies,[129,130]as well as in patients with metastatic renal cell carcinoma in a small phase I trial.[131]
As mentioned in section 3.2, Fong et al.[18]used DCs loaded with a CEA-agonist peptide as a vaccine strategy for patients with advanced CEA tumors. Although only 12 patients were vaccinated, this approach produced clinical responses that correlated with immunologic response to the vaccine. However, the drawback for loading DCs with a particular peptide is that patients are restricted to the HLA haplotype expressed on that peptide. For CTL activation, the MHC I complex on DCs can be loaded with immunogenic peptides that correspond to the patient-specific HLA haplotype.[132]Therefore, patients must be classified by their HLA type, and binding properties of the immunogenic peptides for the specific HLA have to be characterized. One way to avoid this problem is to use TAA encoded by DNA.[133]For this purpose, the patient-specific TAA gene has to be cloned into a vector before transfer into the DC, a labor-intensive and time-consuming process. Additionally, at present, there is no optimal expression system for human DCs.
Another approach is the introduction of mRNA encoding TAA into DCs.[134]RNA can be extracted from a small amount of tumor and amplified in vitro.[135]The mRNA extracted from the tumor is patient-specific and encodes all TAA expressed by the tumor sample. Few studies have used DCs transfected with mRNA.[15,134–137]Among these studies, some used mRNA encoding human CEA.[135,137,138]Transfection of DC with CEA-mRNA performed by lipofection, a technique used to transfer functional genes into a cell, successfully induced CEA-specific CD4+ and CD8+ T lymphocytes in vitro.[135,137,138]Moreover, in mice, specific in vivo CTL response and regression of lung metastases were achieved.[135]These promising results using DCs transfected with TAA-encoding RNA justify further investigations to obtain more experience with this important immunotherapeutic approach. In a recent study by Morse et al.,[17]performed at Duke University Medical Center (Durham, NC, USA), 21 patients with advanced CEA-expressing tumors received vaccinations with in vitro-generated DCs, loaded with an HLA-A2-restricted peptide of CEA to test the safety, feasibility, and clinical response. The DCs were loaded with the CEA peptide CAP-1 and cryopreserved. Groups of between three and six patients received four weekly or biweekly IV infusions of the CAP-1-loaded DCs, which were administered in escalating dose levels of 1 × 107, 3 × 107, and 1 × 108 cells/dose. A subset of the patients in the last group also received intradermal injections of 1 × 106 DCs. There were no toxicities directly related to the treatments. One patient had a minor response, and one had stable disease. Skin punch biopsy at DC injection sites demonstrated pleomorphic infiltrates in the three patients evaluated. The authors concluded that it was safe and feasible to administer cryopreserved DC-expressing CEA to patients with advanced metastatic CEA-expressing tumors.[17]
Rains et al.[139]conducted a study in which 15 patients with advanced colorectal cancer were treated with vaccines prepared from autologous DCs pulsed with tumor RNA and keyhole limpet hemocyanin. Although no radiologic objective responses have been seen to date, no major adverse effects were observed, and in seven patients, CEA levels fell, suggesting some activity of the vaccine. Furthermore, 11 of 13 patients tested developed a positive keyhole limpet hemocyanin skin test.[139]
A recent phase I/II study of active immunotherapy with CEA RNA-pulsed DCs in patients with resected hepatic metastases from adenocarcinoma of the colon has been initiated at Duke University Medical Center (Durham, NC, USA). Patients undergo leukapheresis for up to 4.5 hours to collect DCs. The separated DCs are then pulsed with CEA RNA. Patients receive CEA RNA-pulsed DCs IV every 2 weeks for a total of four doses. Patients then undergo a second leukapheresis 2 weeks after the last DC infusion to obtain specimens for immunologic tests. Patients with extra samples of DCs available may receive additional doses of CEA RNA-pulsed DCs every 2 months in the absence of unacceptable toxicity. Patients are followed up at weeks 12, 24, 36, and 48, and every 6 months thereafter.
4. Conclusion
Recent advances in the field of tumor immunology have provided insight into the mechanisms by which T cells can be activated against TAAs. The preclinical proofs of principle have allowed for the translation of this work into clinical vaccine trials for the treatment of colorectal cancer. A number of different approaches have now been examined in the clinic, which include the modification of whole tumor cell vaccine to a number of different strategies for the delivery of TAAs as vaccines (table III).
Approaches that incorporate ‘off-the-shelf’ technology avoid many of the technical hurdles presented by the more time-consuming manipulation of patient tumors or blood cells. As our knowledge of this field continues to expand, the combination of one or more of these approaches, as well as the integration of vaccines with more traditional therapeutics such as chemotherapy (some of which may also decrease suppressor cell activity) and radiation (which may also upregulate MHC, fas or TAA) may improve upon the success for the treatment of colorectal cancer if given at the proper dose and schedule. Further studies of dose and scheduling regimens will help to determine how vaccines can be combined with traditional anticancer therapeutic modalities.
References
American Cancer Society. Cancer facts and figures: 2001. A lanta (GA): American Cancer Society, 2001
Saltz LB, Cox JV, Blanke C, et al. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. N Engl J Med 2000; 343: 905–14
Fuchs EJ, Matzinger P. Is cancer dangerous to the immune system? Semin Immunol 1996; 8: 271–80
Davis ID, Jefford M, Parente P, et al. Rational approaches to human cancer immunotherapy. J Leukoc Biol 2003 Jan; 73(1): 3–29
Smyth JM, Godfrey DI, Trapani JA. A fresh look at tumor immunosurveillance and immunotherapy. Nat Immunol 2001; 2: 293–9
Vermerken JB, Claessen AM, van Tinteren H, et al. Active specific immunotherapy for stage II and stage III human colon cancer: a randomised trial. Lancet 1999; 353: 345–50
HooverJr HC, Brandhorst JS, Peters LC, et al. Adjuvant active specific immunotherapy for human colorectal cancer: 6.5-year median follow-up of a phase III prospectively randomized trial. J Clin Oncol 1993; 11: 390–9
Harris JE, Ryan L, Hoover Jr HC, et al. Adjuvant active specific immunotherapy of stage II and III colon cancer with an autologous tumor cell vaccine: ECOG study E5283. J Clin Oncol 2000; 18: 148–57
Calmette A. Preventive vaccination against tuberculosis with BCG. Proc R Soc Med 1931; 24: 85–94
BastJr RC, Zbar B, Borsos T, et al. BCG and cancer (first of two parts). N Engl J Med 1974 Jun 20; 290(25): 1413–20
Wittig B, Märten A, Dorbic T, et al. Therapeutic vaccination against metastatic carcinoma by expression-modulated and immunomodified autologous tumor cells: a first clinical phase I/II trial. Hum Gene Ther 2001; 12(3): 267–78
Habal N, Gupta RK, Bilchik AJ, et al. CancerVax, an allogeneic tumor cell vaccine, induces specific humoral and cellular immune responses in advanced colon cancer. Ann Surg Oncol 2001 Jun; 8(5): 389–401
Marshall JL, Hawkins MJ, Tsang KY, et al. Phase I study in cancer patients of a replication-defective avipox recombinant vaccine that expresses human carcinoembryonic antigen. J Clin Oncol 1999; 17: 332–7
Marshall JL, Hoyer RJ, Toomey MA, et al. Phase I study in advanced cancer patients of a diversified prime-and-boost vaccination protocol using recombinant vaccinia virus and recombinant nonreplicating avipox virus to elicit anti-carcinoembryonic antigen immune responses. J Clin Oncol 2000; 18(23): 3964–73
Nair SK, Hull S, Coleman D. Induction of carcinoembryonic antigen (CEA)-specific cytotoxic T-lymphocyte responses in vitro using autologous dendritic cells loaded with CEA peptide or CEA RNA in patients with metastatic malignancies expressing CEA. Int J Cancer 1999; 82: 121–4
von Mehren M, Arlen P, Gulley J, et al. The influence of granulocyte macrophage colony-stimulating factor and prior chemotherapy on the immunological response to a vaccine (ALVAC-CEA B7.1) in patients with metastatic carcinoma. Clin Cancer Res 2001; 7: 1181–91
Morse MA, Deng Y, Coleman D, et al. A Phase I study of active immunotherapy with carcinoembryonic antigen peptide (CAP-1)-pulsed, autologous human cultured dendritic cells in patients with metastatic malignancies expressing carcinoembryonic antigen. Clin Cancer Res 1999; 5: 1331–8
Fong L, Hou Y, Rivas A, et al. Altered peptide ligand vaccination with Flt3 ligand expanded dendritic cells for tumor immunotherapy. Proc Natl Acad Sci U S A 2001; 98: 8809–14
Fagerberg J, Steinitz M, Wigzell H, et al. Human anti-idiotypic antibodies induced a humoral and cellular immune response against a colorectal carcinoma- associated antigen in patients. Proc Natl Acad Sci USA 1995; 92: 4773–7
Denton GW, Durrant LG, Hardcastle JD, et al. Clinical outcome of colorectal cancer patients treated with human monoclonal anti-idiotypic antibody. Int J Cancer 1994; 57: 10–4
Maxwell-Armstrong CA, Durrant LG, Buckley TJ, et al. Randomized double-blind phase II survival study comparing immunization with the anti-idiotypic monoclonal antibody 105AD7 against placebo in advanced colorectal cancer. Br J Cancer 2001 Jun 1; 84(11): 1443–6
Pervin S, Chakraborty M, Bhattacharya-Chatterjee M, et al. Induction of antitumor immunity by an anti-idiotype antibody mimicking carcinoembryonic antigen. Cancer Res 1997; 57: 728–34
Ruffini PA, Kwak LW. Immunotherapy of multiple myeloma. Semin Hematol 2001; 38: 260–7
Esserman LJ, Lopez T, Montes R, et al. Vaccination with the extracellular domain of p185neu prevents mammary tumor development in neu transgenic mice. Cancer Immunol Immunother 1999; 47: 337–42
Gansbacher B, Zier K, Cronin K, et al. Retroviral gene transfer induced constitutive expression of interleukin-2 or interferon-gamma in irradiated human melanoma cells. Blood 1992 Dec 1; 80(11): 2817–25
McBride WH, Thacker JD, Comora S, et al. Genetic modification of a murine fibrosarcoma to produce interleukin 7 stimulates host cell infiltration and tumor immunity. Cancer Res 1992 Jul 15; 52(14): 3931–7
Schadendorf D, Czarnetzki BM, Wittig B. Interleukin-7, interleukin-12, and GM-CSF gene transfer in patients with metastatic melanoma. J Mol Med 1995 Sep; 73(9): 473–7
Finke S, Trojaneck B, Moller P. Increase of cytotoxic sensitivity of primary human melanoma cells transfected with the interleukin-7 gene to autologous and allogeneic immunologic effector cells. Cancer Gene Ther 1997 Jul–Aug; 4(4): 260–8
Hobeika AC, Clay TM, Mosca PJ, et al. Quantitating therapeutically relevant T-cell responses to cancer vaccines. Crit Rev Immunol 2001; 21: 287–97
Hodge JW, Abrams S, Schlom J, et al. Induction of antitumor immunity by recombinant vaccinia viruses expressing B7-1 or B7-2 costimulatory molecules. Cancer Res 1994; 54: 5552–5
Jaffee EM, Hruban RH, Biedrzycki B, et al. Novel allogeneic granulocyte-macrophage colony-stimulating factor-secreting tumor vaccine for pancreatic cancer: a phase I trial of safety and immune activation. J Clin Oncol 2001; 19: 145–56
van der Burg SH, Menon AG, Redeker A, et al. Induction of p53-specific immune responses in colorectal cancer patients receiving a recombinant ALVAC-p53 candidate vaccine. Clin Cancer Res 2002; 8: 1019–27
Sadanaga N, Nagashima H, Mashino K, et al. Dendritic cell vaccination with MAGE peptide is a novel therapeutic approach for gastrointestinal carcinomas. Clin Cancer Res 2001; 7: 2277–84
Khleif SN, Abrams SI, Hamilton JM, et al. A phase I vaccine trial with peptides reflecting ras oncogene mutations of solid tumors. J Immunother 1999; 22: 155–65
Gjertsen MK, Bjørheim J, Saeterdal I, et al. Cytotoxic CD4+ and CD8+ T lymphocytes, generated by mutant p21-ras (12Val) peptide vaccination of a patient, recognize 12Val-dependent nested epitopes present within the vaccine peptide and kill autologous tumour cells carrying this mutation. Int J Cancer 1997; 72: 784–90
Hoon DSB, Irie RF. Current status of human melanoma vaccines: can they control malignant melanoma? BioDrugs 1997; 7: 66–84
Morton DL, Foshag LJ, Hoon DSB, et al. Prolongation of survival in metastatic melanoma after active specific immunotherapy with a new polyvalent melanoma vaccine. Ann Surg 1992; 216: 463–82
Morton DL, Hoon DSB, Nizze JA, et al. Polyvalent melanoma vaccine improves survival of patients with metastatic melanoma. Ann N Y Acad Sci 1993; 690: 120–34
Takahashi T, Johnson TD, Nishinaka Y, et al. IgM anti-ganglioside antibodies induced by melanoma cell vaccine correlate with survival of melanoma patients. J Invest Dermatol 1999; 112: 101–5
Hoon DSB, Morisaki T, Uchiyama A, et al. Augmentation of T-cell response with a melanoma cell vaccine expressing specific HLA-A antigens. Ann N Y Acad Sci 1993; 690: 343–5
Hsueh EC, Famatiga E, Gupta RK, et al. Enhancement of complement-dependent cytotoxicity by polyvalent melanoma cell vaccine (CancerVax): correlation with survival. Ann Surg Oncol 1998; 5: 595–602
Barth A, Hoon DSB, Foshag LJ, et al. Polyvalent melanoma cell vaccine induces delayed-type hypersensitivity and in-vitro cellular immune response. Cancer Res 1994; 54: 3342–5
Jones RC, Kelley M, Gupta RK, et al. Immune response to polyvalent melanoma cell vaccine in AJCC stage III melanoma: an immunologic survival model. Ann Surg Oncol 1996; 3: 437–45
Hsueh EC, Gupta RK, Qi K, et al. TA90 immune complex predicts survival following surgery and adjuvant vaccine immunotherapy for stage IV melanoma. Cancer J Sci Am 1997; 3: 364–70
Hsueh EC, Gupta RK, Morton DL. Correlation of specific immune responses with survival in melanoma patients with distant metastases receiving polyvalent melanoma cell vaccine. J Clin Oncol 1998; 16: 2913–20
Wallack MK, Sivanandham M, Balch CM, et al. Surgical adjuvant active specific immunotherapy for patients with stage III melanoma: the final analysis of data from a phase III, randomized, double-blind, multicenter vaccinia melanoma oncolysate trial. J Am Coll Surg 1998; 187: 69–77
Hellstrom I, Hellstrom KE, Shepard TH. Cell-mediated immunity against antigens common to human colonic carcinomas and fetal gut epithelium. Int J Cancer 1970; 6: 346–51
Elias EG, Elias LL, Didolkar MS, et al. Cellular immunity in patients with colorectal adenocarcinoma measured by autologous leukocyte migration inhibition. Cancer 1977 Aug; 40(2): 687–92
Hollinshead A, Elias EG, Arlen M, et al. Specific active immunotherapy in patients with adenocarcinoma of the colon utilizing TAAs: a phase I clinical trial. Cancer 1985 Aug 1; 56(3): 480–9
Moss B. Genetically engineered poxviruses for recombinant gene expression, vaccination, and safety. Proc Natl Acad Sci U S A 1996; 93: 11341–8
Paoletti E. Applications of pox virus vectors to vaccination: an update. Proc Natl Acad Sci U S A 1996; 93: 11349–53
Carroll MW, Moss B. Poxviruses as expression vectors. Curr Opin Biotechnol 1997; 8: 573–7
Rolph MS, Ramshaw LA. Recombinant viruses as vaccines and immunological tools. Curr Opin Immunol 1997; 9: 517–24
Weiskirch LM, Paterson Y. Listeria monocytogenes: a potent vaccine vector for neoplastic and infectious disease. Immunol Rev 1997; 158: 159–69
Kaufmann SH, Hess J. Impact of intracellular location of and antigen display by intracellular bacteria: implications for vaccine development. Immunol Lett 1999; 65: 81–4
Fenner F, Henderson DA, Arita I, et al. Smallpox and its eradication. Geneva: World Health Organization, 1988
Juillard V, Villefroy P, Godfrin D, et al. Long-term humoral and cellular immunity induced by a single immunization with replication-defective adenovirus recombinant vector. Eur J Immunol 1995; 25: 3467–73
Chen PW, Wang M, Bronte V, et al. Therapeutic antitumor response after immunization with a recombinant adenovirus encoding a model tumor-associated antigen. J Immunol 1996; 156: 224–31
Xiang ZQ, Yang Y, Wilson JM, et al. A replication-defective human adenovirus recombinant serves as a highly efficacious vaccine carrier. Virology 1996; 219: 220–7
Rosenberg SA, Zhai Y, Yang JC, et al. Immunizing patients with metastatic melanoma using recombinant adenoviruses encoding MART-1 or gp100 melanoma antigens. J Natl Cancer Inst 1998; 90: 1894–900
Irvine K, et al. Comparison of a CEA-recombinant vaccinia virus, purified CEA, and an anti-idiotype antibody bearing the image of a CEA epitope in the treatment and prevention of CEA-expressing tumors. Vaccine Res 1993; 2: 79–94
Kass E, Schlom J, Thompson J, et al. Induction of protective host immunity to carcinoembryonic antigen (CEA), a self-antigen in CEA transgenic mice, by immunizing with a recombinant vaccinia-CEA virus. Cancer Res 1999; 59: 676–83
Bernards R, Destree A, McKenzie S, et al. Effective tumor immunotherapy directed against an oncogene-encoded product using a vaccinia virus vector. Proc Natl Acad Sci U S A 1987; 19: 6854–8
Tsang KY, Zaremba S, Nieroda CA, et al. Generation of human eytotoxic T cells specific for human carcinoembryonic antigen epitopes from patients immunized with recombinant vaccinia-CEA vaccine. J Natl Cancer Inst 1995; 87: 982–90
Cole DJ, Wilson MC, Baron PL, et al. Phase I study of recombinant CEA vaccinia virus vaccine with post vaccination CEA peptide challenge. Hum Gene Ther 1996 Jul 10; 7(11): 1381–94
Zhu MZ, Marshall J, Cole D, et al. Specific cytolytic T-ceii responses to human CEA from patients immunized with recombinant avipox-CEA vaccine. Clin Cancer Res 2000; 6: 24–33
Restifo NP, Rosenberg SA. Developing recombinant and synthetic vaccines for the treatment of melanoma. Curr Opin Oncol 1999; 11: 50–7
Bei R, Kantor J, Kashmiri SV, et al. Enhanced immune responses and anti-tumor activity by baculovirus recombinant carcinoembryonic antigen (CEA) in mice primed with the recombinant vaccinia CEA. J Immunother Emphasis Tumor Immunol 1994; 16: 275–82
Hodge JW, McLaughlin JP, Kantor JA, et al. Diversified prime and boost protocols using recombinant vaccinia virus and recombinant non-replicating avian pox virus to enhance T-cell immunity and antitumor responses. Vaccine 1997; 15: 759–68
Irvine KR, Chamberlain RS, Shulman EP, et al. Enhancing efficacy of recombinant anticancer vaccines with prime/boost regimens that use two different vectors. J Natl Cancer Inst 1997; 89: 1595–601
Murata K, Garcia-Sastre A, Tsuji M, et al. Characterization of in vivo primary and secondary CD8+ T cell responses induced by recombinant influenza and vaccinia viruses. Cell Immunol 1996; 173: 96–107
Bednarek MA, Sauma SY, Gammon MC, et al. The minimum peptide epitope from the influenza virus matrix protein: extra and intracellular loading of HLA-A2. J Immunol 1991; 147(12): 4047–53
Slack R, Ley L, Chang P, et al. Association between CEA-specific T cell responses (TCR) following treatment with vaccinia-CEA (V) and Alvac-CEA (A) and survival in patients (pts) with CEA-bearing cancers [abstract no. 1086]. 37th Annual Meeting of the American Society of Clinical Oncology; 2001 May 12–15; San Francisco
Schwartz RH. Costimulation of T lymphocytes: the role of CD28, CTLA-4, and B7/BB1 in interleukin-2 production and immunotherapy. Cell 1992; 71: 1065–8
Chen L, Ashe S, Brady WA, et al. Costimulation of antitumor immunity by the B7 counterreceptor for the T lymphocyte molecules CD28 and CTLA-4. Cell 1992; 71: 1093–102
Freeman GJ, Freedman AS, Segil JM, et al. B7, a new member of the Ig superfamily with unique expression on activated and neoplastic B cells. J Immunol 1989; 143: 2714–22
Freeman GJ, Gray GS, Gimmi CD, et al. Structure, expression, and T cell costimulatory activity of the murine homologue of the human B lymphocyte activation antigen B7. J Exp Med 1991; 174: 625–31
Hellstrom KE, Hellstrom I, Linsley P, et al. On the role of costimulation in tumor immunity. Ann N Y Acad Sci 1993; 690: 225–30
Hellstrom I, Hellstrom KE. Tumor immunology: an overview. Ann N Y Acad Sci 1993; 690: 24–31
Gregory CD, Murray RJ, Edwards CE, et al. Down-regulation of cell adhesion molecules LFA-3 and ICAM-1 in Epstein-Barr virus-positive Burkitt’s lymphoma underlies tumor cell escape from virus-specific T cell surveillance. J Exp Med 1988; 167: 1811–24
Damle NK, Klussman K, Linsley PS, et al. Differential costimulatory effects of adhesion molecules B7, ICAM-1, LFA-3, and VCAM-1 on resting and antigenprimed CD4+ T lymphocytes. J Immunol 1992; 148: 1985–92
Townsend SE, Allison JP. Tumor rejection after direct costimulation of CD8+ T cells by B7-transfected melanoma cells. Science 1993; 259: 368–70
Chen L, Linsley PS, Hellstrom KE. Costimulation of T cells for tumor immunity. Immunol Today 1993; 14: 483–6
von Mehren M, Arlen P, Tsang KY, et al. Pilot study of a dual gene recombinant avipox vaccine containing both carcinoembryonic antigen and B7.1 transgenes in patients with recurrent CEA-expressing adenocarcinomas. Clin Cancer Res 2000; 6: 2219–28
Springer TA. Adhesion receptors of the immune system. Nature 1990; 346: 425–34
Lorenz MG, Kantor JA, Schlom J, et al. Induction of anti-tumor immunity elicited by tumor cells expressing a murine LFA-3 analog via a recombinant vaccinia virus. Hum Gene Ther 1999; 10: 623–31
Uzendoski K, Kantor JA, Abrams SI, et al. Construction and characterization of a recombinant vaccinia virus expressing murine intercellular adhesion molecule-1: induction and potentiation of antitumor responses. Hum Gene Ther 1997; 8: 851–60
Grosenbach DW, Barrientos JC, Schlom J, et al. Synergy of vaccine strategies to amplify antigen-specific immune responses and anti-tumor effects. Cancer Res 2001; 61: 4497–505
Hodge JW, Sabzevari H, Yafal AG, et al. A triad of costimulatory molecules synergize to amplify T-cell activation. Cancer Res 1999; 59: 5800–7
Marshall JL, Rizvi N, Fox E, et al. A Phase I study of sequential vaccinations with fowlpox-CEA (6D)-TRICOM (B7/ICAM/LFA3) alone, and in combination with vaccinia-CEA (6D)-TRICOM and GM-CSF in patients with CEA expressing carcinomas [abstract]. EORT/AACR Meeting; 2001 Oct 29–Nov 2; Miami
Gulley J, Chen AP, Dahut W, et al. A Phase I study of a vaccine using recombinant vaccinia virus expressing PSA (rV-PSA) in patients with metastatic androgen-independent prostate cancer. Prostate 2002; 53: 109–17
Abrams SI, Khleif SN, Bergmann-Leitner ES, et al. Generation of stable CD4+ and CD8+ T cell lines from patients immunized with ras oncogene-derived peptides reflecting codon 12 mutations. Cell Immunol 1997; 182: 137–51
Gjertsen MK, Bakka A, Breivik J, et al. Ex vivo ras peptide vaccination in patients with advanced pancreatic cancer: results of a phase I/II study. Int J Cancer 1996; 65: 450–3
Disis ML, Grabstein KH, Sleath PR, et al. Generation of immunity to the HER-2/neu oncogenic protein in patients with breast and ovarian cancer using a peptide-based vaccine. Clin Cancer Res 1999; 5: 1289–97
Zaremba S, Barzaga E, Zhu M, et al. Identification of an enhancer agonist cytotoxic T lymphocyte peptide from human carcinoembryonic antigen. J Natl Cancer Inst 1997; 57: 4570–7
Goydos JS, Elder E, Whiteside TL, et al. A phase I trial of a synthetic mucin peptide vaccine: induction of specific immune reactivity in patients with adenocarcinoma. J Surg Res 1996; 63: 298–304
Finn OJ, Jerome KR, Henderson RA, et al. MUC-1 epithelial tumor mucin-based immunity and cancer vaccines. Immunol Rev 1995; 145: 61–89
Dudley ME, Ngo LT, Westwood J, et al. T-cell clones from melanoma patients immunized against an anchor-modified gp100 peptide display discordant effector phenotypes. Cancer J 2000; Mar–Apr; 6(2): 69–77
van Driel WJ, Kenter GG, Fleuren GJ, et al. Immunotherapeutic strategies for cervical squamous carcinoma. Hematol Oncol Clin North Am 1999; 13: 259–73
Marchand M, van Baren N, Weynants P, et al. Tumor regressions observed in patients with metastatic melanoma treated with an antigenic peptide encoded by gene MAGE-3 and presented by HLA- A1. Int J Cancer 1999; 80: 219–30
Jager E, Ringhoffer M, Dienes HP, et al. Granulocyte-macrophage-colony-stimulating factor enhances immune responses to melanoma-associated peptides in vivo. Int J Cancer 1996; 67: 54–62
Rosenberg SA, Yang JC, Schwartzentruber DJ, et al. Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma. Nat Med 1998; 4: 321–7
Salgaller ML, Marincola FM, Cormier JN, et al. Immunization against epitopes in the human melanoma antigen gp100 following patient immunization with synthetic peptides. Cancer Res 1996; 56: 4749–57
Murphy GP, Elgamal AA, Su SL, et al. Current evaluation of the tissue localization and diagnostic utility of prostate specific membrane antigen. Cancer 1998; 83: 2259–69
Murphy GP, Tjoa BA, Simmons SJ, et al. Infusion of dendritic cells pulsed with HLA-A2-specific prostate- specific membrane antigen peptides: a phase II prostate cancer vaccine trial involving patients with hormone-refractory metastatic disease. Prostate 1999; 38: 73–8
Jerne NK. Towards a network theory of the immune system. Ann Immunol (Paris) 1974; 125: 373–89
Cerny J, Hiemaux J. Concept of idiotypic network: description and functions. In: Cerny J, Hiernaux J, editors. Idiotypic network and diseases. Washington,DC: American Society for Microbiology, 1990: 12–30
Chakraborty M, Mukerjee S, Foon K, et al. Induction of human breast cancer-specific antibody responses in cynomolgous monkeys by a murine monoclonal anti-idiotype antibody. Cancer Res 1995; 55: 1525–30
Chatterjee M, Foon K, Köhler H. Idiotypic antibody immunotherapy of cancer. Cancer Immunol Immunother 1994; 38: 75–82
Jefferis R. What is an idiotype? Immunol Today 1993; 14: 119–21
Varela F, Coutinho A. second generation immune networks. Immunol Today 1991; 12: 159–66
Foon K, Chakraborty M, John W, et al. Immune response to the carcinoembryonic antigen in patients treated with an anti-idiotype vaccine. J Clin Investig 1995; 96: 334–42
Foon K, Bhattacharya-Chatterjee M. Idiotype vaccines in the clinic [letter]. Nat Med 1998; 4: 870
Herlyn D, Zaloudik J, Somasundaram R, et al. Anti-idiotype vaccine in colorectal cancer patients. Hybridoma 1993; 12: 515–20
Herlyn D, Benden A, Kane M, et al. Anti-idiotype cancer vaccines: pre-clinical and clinical studies. In Vivo 1991; 5: 615–24
Herlyn D, Harris D, Zaloudik J, et al. Immunomodulatory activity of monoclonal anti-idiotypic antibody to anti-colorectal carcinoma antibody CO17-1A in animals and patients. J Immunother Emphasis Tumor Immunol 1994 May; 15(4): 303–11
Durrant LG, Maxwell-Armstrong C, Buckley D, et al. A neoadjuvant clinical trial in colorectai cancer patients of the human anti-idiotypic antibody 105AD7, which mimics CD55. Clin Cancer Res 2000 Feb; 6(2): 422–30
Maxwell-Armstrong CA, Durrant LG, Robins RA, et al. Increased activation of lymphocytes infiltrating primary colorectai cancers following immunisation with the anti-idiotypic monoclonal antibody 105AD7. Gut 1999 Oct; 45(4): 593–8
Amin S, Robins RA, Maxwell-Armstrong CA, et al. Vaccine-induced apoptosis: a novel clinical trial end point? Cancer Res 2000 Jun 15; 60(12): 3132–6
Foon KA, John WJ, Chakraborty M, et al. Clinical and immune responses in advanced colorectai cancer patients treated with anti-idiotype monoclonal antibody vaccine that mimics the carcinoembryonic antigen. Clin Cancer Res 1997; 3: 1267–76
Foon KA, John WJ, Chakraborty M, et al. Clinical and immune responses in resected colon cancer patients treated with anti-idiotype monoclonal antibody vaccine that mimics the carcinoembryonic antigen. J Clin Oncol 1999; 17: 2889–95
Banchereau J, Schuler-Thurner B, Palucka AK, et al. Dendritic cells as vectors for therapy. Cell 2001; 106: 271–4
Steinman RM, Dhodapkar M. Active immunization against cancer with dendritic cells: the near future. Int J Cancer 2001; 94: 459–73
Hsu FJ, Benike C, Fagnoni F, et al. Vaccination of patients with B-cell lymphoma using autologous antigen-pulsed dendritic cells. Nat Med 1996; 2: 52–8
Reichardt VL, Okada CY, Liso A, et al. Idiotype vaccination using dendritic cells after autologous peripheral blood stem cell transplantation for multiple myeloma: a feasibility study. Blood 1999; 93: 2411–9
Nestle FO, Alijagic S, Gilliet M, et al. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat Med 1998; 4: 328–32
Palucka K, Banchereau J. Dendritic cells: a link between innate and adaptive immunity. J Clin Immunol 1999; 19: 12–25
Timmerman JM, Levy R. Dendritic cell vaccines for cancer immunotherapy. Annu Rev Med 1999; 50: 507–29
Tanaka Y, Koido S, Chen D, et al. Vaccination with allogeneic dendritic cells fused to carcinoma cells induces antitumor immunity in MUC1 transgenic mice. Clin Immunol 2001; 101: 192–200
Koido S, Tanaka Y, Chen D, et al. The kinetics of in vivo priming of CD4 and CD8 T cells by dendritic/tumor fusion cells in MUC1-transgenic mice. J Immunol 2002; 168: 2111–7
Kugler A, Stuhler G, Waiden P, et al. Regression of human metastatic renal cell carcinoma after vaccination with tumor cell-dendritic cell hybrids. Nat Med 2000; 6: 332–6
Eisenbach L, Bar-Haim E, El-Shami K. Antitumor vaccination using peptide based vaccines. Immunol Lett 2000; 74: 27–34
Akbari O, Panjwani N, Garcia G, et al. DNA vaccination: transfection and activation of dendritic cells as key events for immunity. J Exp Med 1999; 189: 169–78
Boczkowski D, Nair SK, Snyder D, et al. Dendritic cells pulsed with RNA are potent antigen-presenting cells in vitro and in vivo. J Exp Med 1996; 184: 465–72
Boczkowski D, Nair SK, Nam JH. Induction of tumor immunity and cytotoxic T lymphocyte responses using dendritic cells transfected with messenger RNA amplified from tumor cells. Cancer Res 2000; 60: 1028–34
Koido S, Kashiwaba M, Chen D, et al. Induction of antitumor immunity by vaccination of dendritic cells transfected with MUC1 RNA. J Immunol 2000; 165: 5713–9
Nair SK, Boczkowski D, Morse M, et al. Induction of primary carcinoembryonic antigen (CEA)-specific cytotoxic T lymphocytes in vitro using human dendritic cells transfected with RNA. Nat Biotechnol 1998; 16: 364–9
Horig H, Lee DS, Conkright W, et al. Phase I clinical trial of a recombinant canarypoxvirus (ALVAC) vaccine expressing human carcinoembryonic antigen and the B7.1 co-stimulatory molecule. Cancer Immunol Immunother 2000 Nov; 49(9): 504–14
Rains N, Cannan RJ, Chen W, et al. Development of a dendritic cell (DC)-based vaccine for patients with advanced colorectal cancer. Hepatogastroenterology 2001 Mar–Apr; 48(38): 347–51
Acknowledgments
The authors thank Debra Weingarten for her editorial assistance in the preparation of this manuscript. No sources of funding were used to assist in the preparation of this manuscript. The authors have no conflicts of interest that are directly relevant to the content of this review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Arlen, P.M., Gulley, J.L. therapeutic vaccines for colorectal cancer. Am J Cancer 3, 299–316 (2004). https://doi.org/10.2165/00024669-200403050-00004
Published:
Issue Date:
DOI: https://doi.org/10.2165/00024669-200403050-00004