Abstract
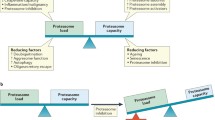
Proteasome inhibition is a new approach under investigation for cancer therapy. Protein degradation by the proteasome is a fundamental metabolic process. Inhibition of the proteasome has been shown to result in cell-cycle arrest and programmed cell death.
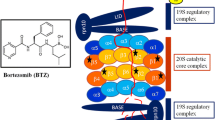
Bortezomib (Velcade®; formerly PS-341, Millennium Pharmaceuticals, Inc, Cambridge, Massachusetts) is the first proteasome inhibitor to enter clinical trials and is approved for the treatment of multiple myeloma in patients who have received at least two prior therapies and progressed on their last therapy. It is also being investigated for the treatment of a range of other cancers, both solid and hematologic. In preclinical studies, bortezomib causes apoptosis in cancer cells and seems to enhance the cytotoxicity of other conventional tumoricidal agents in human tumor cell lines, murine tumor models, and human xenograft models. In addition to targeting cancer cells directly, there is growing evidence that proteasome inhibition interferes with protective interactions between cancer cells and the bone marrow and may also act to prevent tumor-associated angiogenesis.
Preliminary data suggest that bortezomib inhibits proteasome activity in a dose-dependent and reversible manner, and has manageable toxicities. A phase II trial in relapsed and refractory multiple myeloma patients indicated that patients experienced substantial anticancer activity when treated with bortezomib monotherapy.




Similar content being viewed by others
References
Hideshima T, Richardson P, Chauhan D, et al. The proteasome inhibitor PS-341 inhibits growth, induces apoptosis, and overcomes drag resistance in human multiple myeloma cells. Cancer Res 2001; 61: 3071–6
LeBlanc R, Catley LP, Hideshima T, et al. Proteasome inhibitor PS-341 inhibits human myeloma cell growth in vivo and prolongs survival in a murine model. Cancer Res 2002; 62: 4996–5000
Orlowski RZ, Eswara JR, Lafond-Walker A, et al. Tumor growth inhibition induced in a murine model of human Burkitt’s lymphoma by a proteasome inhibitor. Cancer Res 1998; 58: 4342–8
Masdehors P, Merle-Beral H, Magdelenat H, et al. Ubiquitin-proteasome system and increased sensitivity of B-CLL lymphocytes to apoptotic death activation. Leuk Lymphoma 2000; 38: 499–504
Delic J, Masdehors P, Omura S, et al. The proteasome inhibitor lactacystin induces apoptosis and sensitizes chemo- and radioresistant human chronic lymphocytic leukaemia lymphocytes to TNF-alpha-initiated apoptosis. Br J Cancer 1998; 77: 1103–7
Kudo Y, Takata T, Ogawa I, et al. p27Kip1 accumulation by inhibition of proteasome function induces apoptosis in oral squamous cell carcinoma cells. Clin Cancer Res 2000; 6: 916–23
An B, Goldfarb RH, Siman R, et al. Novel dipeptidyl proteasome inhibitors overcome Bcl-2 protective function and selectively accumulate the cyclin-dependent kinase inhibitor p27 and induce apoptosis in transformed, but not normal, human fibroblasts. Cell Death Differ 1998; 5: 1062–75
Soligo D, Servida F, Delia D, et al. The apoptogenic response of human myeloid leukaemia cell lines and of normal and malignant haematopoietic progenitor cells to the proteasome inhibitor PSI. Br J Haematol 2001; 113: 126–35
Shah SA, Potter MW, McDade TP, et al. 26S proteasome inhibition induces apoptosis and limits growth of human pancreatic cancer. J Cell Biochem 2001; 82: 110–22
Bold RJ, Virudachalam S, McConkey DJ. Chemosensitization of pancreatic cancer by inhibition of the 26S proteasome. J Surg Res 2001; 100: 11–7
Frankel A, Man S, Elliott P, et al. Lack of multicellular drug resistance observed in human ovarian and prostate carcinoma treated with the proteasome inhibitor PS-341. Clin Cancer Res 2000; 6: 3719–28
Adams J, Palombella VJ, Sausville EA, et al. Proteasome inhibitors: a novel class of potent and effective antitumor agents. Cancer Res 1999; 59: 2615–22
Sunwoo JB, Chen Z, Dong G, et al. Novel proteasome inhibitor PS-341 inhibits activation of nuclear factor-kappaB, cell survival, tumor growth, and angiogenesis in squamous cell carcinoma. Clin Cancer Res 2001; 7: 1419–28
CusackJr JC, Liu R, Houston M, et al. Enhanced chemosensitivity to CPT-11 with proteasome inhibitor PS-341: implications for systemic nuclear factor-kappaB inhibition. Cancer Res 2001; 61: 3535–40
Russo SM, Tepper JE, Baldwin AS, et al. Enhancement of radiosensitivity by proteasome inhibition: Implications for a role of NF-κB. Int J Radiat Oncol Biol Phys 2001; 50: 183–93
Pervan M, Pajonk F, Sun JR, et al. Molecular pathways that modify tumor radiation response. Am J Clin Oncol 2001; 24: 481–5
Pink M, Pien CS, Worland P, et al. PS-341 enhances chemotherapeutic effect in human xenograft models [abstract no.787]. Proc Am Assoc Cancer Res 2002; 43: 158
American Cancer Society. Cancer facts & figures 2002. A lanta (GA): American Cancer Society Inc, 2002
Oken MM, Harrington DP, Abramson N, et al. Comparison of melphalan and prednisone with vincristine, carmustine, melphalan, cyclophosphamide, and prednisone in the treatment of multiple myeloma: results of Eastern Cooperative Oncology Group Study E2479. Cancer 1997; 79: 1561–7
Zwickl P, Voges D, Baumeister W. The proteasome: a macromolecular assembly designed for controlled proteolysis. Philos Trans R Soc Lond B Biol Sci 1999; 354: 1501–11
Palombella VJ, Rando OJ, Goldberg AL, et al. The ubiquitin-proteasome pathway is required for processing the NF- kappa B1 precursor protein and the activation of NF-kappa B. Cell 1994; 78: 773–85
Pagano M, Tarn SW, Theodoras AM, et al. Role of the ubiquitin-proteasome pathway in regulating abundance of the cyclin-dependent kinase inhibitor p27. Science 1995; 269: 682–5
Cayrol C, Ducommun B. Interaction with cyclin-dependent kinases and PCNA modulates proteasome-dependent degradation of p21. Oncogene 1998; 17: 2437–44
Nesbit CE, Tersak JM, Prochownik EV. MYC oncogenes and human neoplastic disease. Oncogene 1999; 18: 3004–16
Clurman BE, Sheaff RJ, Thress K, et al. Turnover of cyclin E by the ubiquitinproteasome pathway is regulated by cdk2 binding and cyclin phosphorylation. Genes Dev 1996; 10: 1979–90
Tatebe H, Yanagida M. Cut8, essential for anaphase, controls localization of 26S proteasome, facilitating destruction of cyclin and Cut2. Curr Biol 2000; 10: 1329–38
Chadebech P, Brichese L, Baldin V, et al. Phosphorylation and proteasome- dependent degradation of Bcl-2 in mitotic-arrested cells after microtubuie damage. Biochem Biophys Res Commun 1999; 262: 823–7
An WG, Chuman Y, Fojo T, et al. Inhibitors of transcription, proteasome inhibitors, and DNA-damaging drags differentially affect feedback of p53 degradation. Exp Cell Res 1998; 244: 54–60
Masdehors P, Merle-Beral H, Maloum K, et al. Deregulation of the ubiquitin system and p53 proteolysis modify the apoptotic response in B-CLL lymphocytes. Blood 2000; 96: 269–74
Ma MH, Yang HH, Parker KM, et al. The proteasome inhibitor PS-341 markedly enhances sensitivity of multiple myeloma tumor cells to chemotherapeutic agents. Clin Cancer Res 2003; 9(3): 1136–44
Hideshima T, Chauhan D, Podar K, et al. Novel therapies targeting the myeloma cell and its bone marrow microenvironment. Semin Oncol 2001; 28: 607–12
Guzman ML, Neering SJ, Upchurch D, et al. Nuclear factor-kappaB is constitutively activated in primitive human acute myelogenous leukemia cells. Blood 2001; 98: 2301–7
Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem 1998; 67: 425–79
Kisselev AF, Goldberg AL. Proteasome inhibitors: from research tools to drug candidates. Chem Biol 2001; 8: 739–58
DeMartino GN, Slaughter CA. The proteasome, a novel protease regulated by multiple mechanisms. J Biol Chem 1999; 274: 22123–6
Nussbaum AK, Dick TP, Keilholz W, et al. Cleavage motifs of the yeast 20S proteasome β subunits deduced from digests of enolase 1. Proc Natl Acad Sci U S A 1998; 95: 12504–9
Richardson PG, Barlogie B, Berenson J, et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med 2003; 348(26): 2609–17
Furet P, Imbach P, Fuerst P, et al. Structure-based optimisation of 2-aminobenzyl-statine derivatives: potent and selective inhibitors of the chymotrypsin-like activity of the human 20S proteasome. Bioorg Med Chem Lett 2002; 12: 1331–4
MacLaren AP, Chapman RS, Wyllie AH, et al. p53-dependent apoptosis induced by proteasome inhibition in mammary epithelial cells. Cell Death Differ 2001; 8: 210–8
Fan XM, Wong BC, Wang WP, et al. Inhibition of proteasome function induced apoptosis in gastric cancer. Int J Cancer 2001; 93: 481–8
Masdehors P, Omura S, Merle-Beral H, et al. Increased sensitivity of CLL-derived lymphocytes to apoptotic death activation by the proteasome-specific inhibitor lactacystin. Br J Haematol 1999; 105: 752–7
Drexler HC. Activation of the cell death program by inhibition of proteasome function. Proc Natl Acad Sci U S A 1997; 94: 855–60
Kumatori A, Tanaka K, Inamura N, et al. Abnormally high expression of proteasomes in human leukemic cells. Proc Natl Acad Sci U S A 1990; 87: 7071–5
An WG, Hwang SG, Trepel JB, et al. Protease inhibitor-induced apoptosis: accumulation of wt p53, p21WAF1/CIP1, and induction of apoptosis are independent markers of proteasome inhibition. Leukemia 2000; 14: 1276–83
Alkalay I, Yaron A, Hatzubai A, et al. Stimulation-dependent I kappa B alpha phosphorylation marks the NF-kappa B inhibitor for degradation via the ubiquitin-proteasome pathway. Proc Natl Acad Sci U S A 1995; 92: 10599–603
Spencer E, Jiang J, Chen ZJ. Signal-induced ubiquitination of I kappa B alpha by the F-box protein Slimb/beta-TrCP. Genes Dev 1999; 13: 284–94
Winston JT, Strack P, Beer-Romero P, et al. The SCFbeta-TRCP-ubiquitin ligase complex associates specifically with phosphorylated destruction motifs in IkappaBalpha and beta-catenin and stimulates IkappaBalpha ubiquitination in vitro. Genes Dev 1999; 13: 270–83
Chen C, Edelstein LC, Gelinas C. The Rel/NF-kappaB family directly activates expression of the apoptosis inhibitor Bcl-x(L). Mol Cell Biol 2000; 20: 2687–95
Mitsiades N, Mitsiades CS, Poulaki V, et al. Biologic sequelae of nuclear factor-kappaB blockade in multiple myeloma: therapeutic applications. Blood 2002; 99: 4079–86
Wang CY, Mayo MW, Korneluk RG, et al. NF-κB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c- IAP2 to suppress caspase~8 activation. Science 1998; 281: 1680–3
Wang CY, Guttridge DC, Mayo MW, et al. NF-kappaB induces expression of the Bcl-2 homologue A1/Bfl-1 to preferentially suppress chemotherapy-induced apoptosis. Mol Cell Biol 1999; 19: 5923–9
Zong WX, Edelstein LC, Chen C, et al. The prosurvival Bcl-2 homolog Bfl-1/A1 is a direct transcriptional target of NF-kappaB that blocks TNFalpha-induced apoptosis. Genes Dev 1999; 13: 382–7
Ni H, Ergin M, Huang Q, et al. Analysis of expression of nuclear factor kappa B (NF-kappa B) in multiple myeloma: downregulation of NF-kappa B induces apoptosis. Br J Haematol 2001; 115: 279–86
Davis RE, Brown KD, Siebenlist U, et al. Constitutive nuclear factor kappaB activity is required for survival of activated B cell-like diffuse large B cell lymphoma cells. J Exp Med 2001; 194: 1861–74
Kordes U, Krappmann D, Heissmeyer V, et al. Transcription factor NF-κB is constitutively activated in acute lymphoblastic leukemia cells. Leukemia 2000; 14: 399–402
Patel NM, Nozaki S, Shortle NH, et al. Paclitaxel sensitivity of breast cancer cells with constitutively active NF-κB is enhanced by IκBα super-repressor and parthenolide. Oncogene 2000; 19: 4159–69
Arlt A, Vorndamm J, Muerkoster S, et al. Autocrine production of interleukin 1beta confers constitutive nuclear factor kappaB activity and chemoresistance in pancreatic carcinoma cell lines. Cancer Res 2002; 62: 910–6
Lind DS, Hochwald SN, Malaty J, et al. Nuclear factor-kappa B is upregulated in colorectal cancer. Surgery 2001; 130: 363–9
Chen CD, Sawyers CL. NF-kappa B activates prostate-specific antigen expression and is upregulated in androgen-independent prostate cancer. Mol Cell Biol 2002; 22: 2862–70
Berenson JR, Ma HM, Vescio R. The role of nuclear factor-kappaB in the biology and treatment of multiple myeloma. Semin Oncol 2001; 28: 626–33
Pajonk F, Pajonk K, McBride WH. Apoptosis and radiosensitization of hodgkin cells by proteasome inhibition. Int J Radiat Oncol Biol Phys 2000; 47: 1025–32
Giuliano M, Lauricella M, Calvaruso G, et al. The apoptotic effects and synergistic interaction of sodium butyrate and MG132 in human retinoblastoma Y79 cells. Cancer Res 1999; 59: 5586–95
Almond JB, Snowden RT, Hunter A, et al. Proteasome inhibitor-induced apoptosis of B-chronic lymphocytic leukaemia cells involves cytochrome c release and caspase activation, accompanied by formation of an approximately 700 kDa Apaf-1 containing apoptosome complex. Leukemia 2001; 15: 1388–97
Naujokat C, Sezer O, Zinke H, et al. Proteasome inhibitors induced caspase-dependent apoptosis and accumulation of p21WAF1/Cip1 in human immature leukemic cells. Eur J Haematol 2000; 65: 221–36
Wagenknecht B, Hermisson M, Groscurth P, et al. Proteasome inhibitor-induced apoptosis of glioma cells involves the processing of multiple caspases and cytochrome c release. J Neurochem 2000; 75: 2288–97
Teicher BA, Ara G, Herbst R, et al. The proteasome inhibitor PS-341 in cancer therapy. Clin Cancer Res 1999; 5: 2638–45
Hideshima T, Chauhan D, Richardson P, et al. NF-kappa B as a therapeutic target in multiple myeloma. J Biol Chem 2002; 277: 16639–47
Feinman R, Gangurde P, Miller S, et al. Proteasome inhibitor PS-341 inhibits constitutive NF-kappaB activation and bypasses the anti-apoptotic bcl-2 signal in human multiple myeloma cells [abstract]. Blood 2001; 98: 640a
LeBlanc R, Catley LP, Hideshima T, et al. Proteasome inhibitor PS-341 inhibits human multiple myeloma cell growth in vivo and prolongs survival in a murine model. Cancer Res 2002; 62: 4996–5000
Phara L, Tamayo A, Yoshimura LC, et al. Inhibition of constitutive NF-kappa B activation in mantle cell lymphoma B cells leads to induction of cell cycle arrest and apoptosis. J Immunol 2003; 171: 88–95
Williams S, Pettaway C, Song R, et al. Differential effects of the proteasome inhibitor bortezomib on apoptosis and angiogenesis in human prostate tumor xenografts. Mol Cancer Ther 2003; 2: 835–43
Kurland JF, Meyn RE. Protease inhibitors restore radiation-induced apoptosis to Bcl-2-expressing lymphoma cells. Int J Cancer 2001; 96: 327–33
Shain KH, Landowski TH, Dalton WS. The tumor microenvironment as a determinant of cancer cell survival: a possible mechanism for de novo drug resistance. Curr Opin Oncol 2000; 12: 557–63
Damiano JS, Dalton WS. Integrin-mediated drug resistance in multiple myeloma. Leuk Lymphoma 2000; 38: 71–81
Anderson KC, Kyle RA, Dalton WS, et al. Multiple myeloma: new insights and therapeutic approaches. Hematology (Am Soc Hematol Educ Program) 2000, 147-65
Landowski TH, Olashaw NE, Agrawal D, et al. Cell adhesion-mediated drag resistance (CAM-DR) is associated with activation of NF-kappa B (RelB/p50) in myeloma cells. Oncogene 2003; 22: 2417–21
Hideshima T, Chauhan D, Schlossman R, et al. The role of tumor necrosis factor alpha in the pathophysiology of human multiple myeloma: therapeutic applications. Oncogene 2001; 20: 4519–27
Drexler HC, Risau W, Konerding MA. Inhibition of proteasome function induces programmed cell death in proliferating endothelial cells. FASEB J 2000; 14: 65–77
Oikawa T, Sasaki T, Nakamura M, et al. The proteasome is involved in angiogenesis. Biochem Biophys Res Commun 1998; 246: 243–8
Nix D, Pien C, Newman R, et al. Clinical development of a proteasome inhibitor, PS-341, for the treatment of cancer [abstract no. 339]. Proc Am Soc Clin Oncol 2001; 20: 86a
Aghajanian C, Soignet S, Dizon DS, et al. A phase I trial of the novel proteasome inhibitor PS341 in advanced solid tumor malignancies. Clin Cancer Res 2002; 8: 2505–11
Oriowski RZ, Stinchcombe TE, Mitchell BS, et al. Phase I trial of the proteasome inhibitor PS-341 in patients with refractory hematologic malignancies. J Clin Oncol 2002; 20: 4420–7
Papandreou CN, Daliani DD, Nix D, et al. Phase I study of thel proteasome inhibitor bortezomib in patients with advanced solid tumors with observations in androgen-independent prostate cancer. J Clin Oncol 2004; 22: 2108–21
Jagannath S, Barlogie B, Berenson J, et al. A phase II study of two doses of bortezomib in relapsed or refractory myeloma. Br J Haematol 2004; 127(2): 165–72
Ryan DP, Eder JP, Winklemann J, et al. Pharmacokinetic and pharmacodynamic phase I study of PS-341 and gemcitabine in patients with advanced solid tumors [abstract no. 379]. Proc Am Soc Clin Oncol 2002; 21: l9a
Bladé J, Samson D, Reece D, et al. Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation. Myeloma Subcommittee of the European Group for Blood and Marrow Transplant (EBMT). Br J Haematol 1998; 102: 1115–23
Jagannath S, Richardson P, Barlogie B, et al. Phase II trials of bortezomib in combination with dexamethasone in multiple myeloma (MM); assessment of additional benefits to combination in patients with sub-optimal response to bortezomib alone [abstract no. 2341]. Proc Am Soc Clin Oncol 2003; 22(582): 582
Richardson PG, Briemberg H, Jagannath S, et al. Peripheral neuropathy following bortezomib (Veicade™, formerly PS-341) therapy in patients with advanced multiple myeloma (MM): characterization and reversibility. Blood 2003; 102(11): 149a
Berenson JR, Jagannath S, Barlogie B, et al. Experience with long-term therapy using the proteasome inhibitor, bortezomib, in advanced multiple myeloma (MM) [abstract no. 2337]. Proc Am Soc Clin Oncol 2003; 22: 581
Richardson PG, Sonnerveld P, Schuster MW, et al. Bortezomib vs dexamethasone in relapsed multiple myeloma: a phase III randomized study. J Clin Oncol 2004; 22: 5605
National Library of Medicine. ClinicalTrials.gov [online]. Available from URL: http://clinicaltrials.gov [Accessed 2002 May 31]
Acknowledgment
This review was supported by an unrestricted educational grant from Millennium Pharmaceuticals, Inc. Drs Richardson and Anderson have received honoraria for lectures and served on advisory boards for Millennium Pharmaceuticals Inc. Dr Hideshima has no conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Richardson, P.G., Hideshima, T. & Anderson, K.C. Proteasome inhibition. Am J Cancer 3, 271–279 (2004). https://doi.org/10.2165/00024669-200403050-00001
Published:
Issue Date:
DOI: https://doi.org/10.2165/00024669-200403050-00001