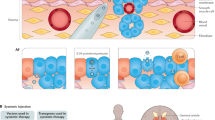
Abstract
Gene therapy for prostate cancer is a relatively new experimental treatment modality and several different therapeutic approaches are being considered. Prostate cancer is the most commonly diagnosed cancer in males and has unique features that make it ideal for gene therapy. Although prostate cancer that is confined to the gland can be cured in many of the patients using local treatments (radical prostatectomy or irradiation therapy), the long-term failure rate of these therapies suggests that cancers can metastasize relatively early in the course of the disease. Once prostate cancer has metastasized there are no curative therapies. The greatest challenge in the treatment of advanced prostate cancer is to access and eliminate metastatic cells. Therefore, successful prostate cancer gene therapy will ultimately require an effective strategy to kill cancer cells both at the site of the primary tumor and at distant metastatic sites.
In this article we review many aspects of gene therapy specifically relevant for prostate cancer. We discuss the unique advantages and disadvantages of nonviral and viral gene delivery systems. Evidence that gene delivery directly into tumors, in situ, is effective for prostate cancer is presented. We provide a broad review of three general approaches or strategies for prostate cancer gene therapy: corrective, cytoreductive, and immuno-modulatory gene therapy. Replacement of the tumor suppressor gene p53 is the best studied example of corrective gene therapy. The cytoreductive gene therapy that has been used most extensively is herpes simplex virus thymidine kinase (HSV-tk) combined with the prodrug ganciclovir. Immunomodulatory gene therapy approaches such as enhancement of cancer cell recognition, e.g. with antigen encoded gene vaccine approaches, and augmentation of the cellular immune response with specific immunomodulatory molecules such as interleukin-12 have the potential to effectively treat both local prostate cancer and distant metastases.




Similar content being viewed by others
References
American Cancer Society. Cancer facts & figures 2002 [online]. Available from URL: http://www.cancer.org/eprise/main/docroot/STT/content/STT_lx_-Cancer_Facts_Figures_2002 [Accessed 2002 May 24]
Ben-Josef E, Shamsa F, Forman JD. Predicting the outcome of radiotherapy for prostate carcinoma: a model-building strategy. Cancer 1998; 82(7): 1334–42
Kattan MW, Wheeler TM, Scardino PT. Postoperative nomogram for disease recurrence after radical prostatectomy for prostate cancer. J Clin Oncol 1999; 17(5): 1499–507
Moul JW, Connelly RR, Lubeck DP, et al. Predicting risk of prostate specific antigen recurrence after radical prostatectomy with the Center for Prostate Disease Research and Cancer of the Prostate Strategic Urologic Research Endeavor databases. J Urol 2001; 166(4): 1322–7
Han M, Partin AW. Nomograms for clinically localized prostate cancer: part I: radical prostatectomy. Semin Urol Oncol 2002; 20(2): 123–30
Ross PL, Gerigk C, Gonen M, et al. Comparisons of nomograms and urologists’ predictions in prostate cancer. Semin Urol Oncol 2002; 20(2): 82–8
Kelly WK, Slovin SF. Chemotherapy for androgen-independent prostate cancer: myth or reality. Curr Oncol Rep 2000; 2(5): 394–401
Steele FR, Aguilar-Cordova E. Cabo II: immunology and gene therapy. Mol Ther 2002; 5 (5 Pt 1): 486–91
Wilson JM. Adenoviruses as gene-delivery vehicles. N Engl J Med 1996; 334(18): 1185–7
Miller AD. Retroviral vectors. Curr Top Microbiol Immunol 1992; 158: 1–24
Latchman DS. Gene delivery and gene therapy with herpes simplex virus-based vectors. Gene 2001; 264(1): 1–9
Mastrangelo MJ, Eisenlohr LC, Gomella L, et al. Poxvirus vectors: orphaned and underappreciated. J Clin Invest 2000; 105(8): 1031–4
Rabinowitz JE, Samulski RJ. Building a better vector: the manipulation of AAV virions. Virology 2000; 278(2): 301–8
Coffey MC, Strong JE, Forsyth PA, et al. Reovirus therapy of tumors with activated Ras pathway. Science 1998; 282(5392): 1332–4
Yu D, Chen D, Chiu C, et al. Prostate-specific targeting using PSA promoter-based lentiviral vectors. Cancer Gene Ther 2001; 8(9): 628–35
Andriani F, Nan B, Yu J, et al. Use of the probasin promoter ARR2PB to express Bax in androgen receptor-positive prostate cancer cells. J Natl Cancer Inst 2001; 93(17): 1314–24
Galanis E, Vile R, Russell SJ. Delivery systems intended for in vivo gene therapy of cancer: targeting and replication competent viral vectors. Crit Rev Oncol Hematol 2001; 38(3): 177–92
Shalev M, Kadmon D, Teh BS, et al. Suicide gene therapy toxicity after multiple and repeat injections in patients with localized prostate cancer. J Urol 2000; 163(6): 1747–50
Miles BJ, Shalev M, Aguilar-Cordova E, et al. Prostate-specific antigen response and systemic T cell activation after in situ gene therapy in prostate cancer patients failing radiotherapy. Hum Gene Ther 2001; 12(16): 1955–67
Teh BS, Aguilar-Cordova E, Kernen K, et al. Phase I/II trial evaluating combined radiotherapy and in situ gene therapy with or without hormonal therapy in the treatment of prostate cancer: a preliminary report. Int J Radiat Oncol Biol Phys 2001; 51(3): 605–13
Martiniello-Wilks R, Garcia-Aragon J, Daja MM, et al. In vivo gene therapy for prostate cancer: preclinical evaluation of two different enzyme-directed prodrug therapy systems delivered by identical adenovirus vectors. Hum Gene Ther 1998; 9(11): 1617–26
Li Y, McCadden J, Ferrer F, et al. Prostate-specific expression of the diphtheria toxin A chain (DT-A): studies of inducibility and specificity of expression of prostate-specific antigen promoter-driven DT-A adenoviral-mediated gene transfer. Cancer Res 2002; 62(9): 2576–82
Koeneman KS, Kao C, Ko SC, et al. Osteocalcin-directed gene therapy for prostate-cancer bone metastasis. World J Urol 2000; 18(2): 102–10
Pramudji C, Shimura S, Ebara S, et al. In situ prostate cancer gene therapy using a novel adenoviral vector regulated by the caveolin-1 promoter. Clin Cancer Res 2001; 7: 4272–9
Douglas JT, Rogers BE, Rosenfeld ME, et al. Targeted gene delivery by tropism-modified adenoviral vectors. Nat Biotechnol 1996; 14(11): 1574–8
Dmitriev I, Krasnykh V, Miller CR, et al. An adenovirus vector with genetically modified fibers demonstrates expanded tropism via utilization of a coxsack-ievirus and adenovirus receptor-independent cell entry mechanism. J Virol 1998; 72(12): 9706–13
Magnusson MK, See Hong S, Henning P, et al. Genetic retargeting of adenovirus vectors: functionality of targeting ligands and their influence on virus viability. J Gene Med 2002; 4(4): 356–70
Rodriguez R, Schuur ER, Lim HY, et al. Prostate attenuated replication competent adenovirus (ARCA) CN706: a selective cytotoxic for prostate-specific antigen-positive prostate cancer cells. Cancer Res 1997; 57(13): 2559–63
Yu DC, Sakamoto GT, Henderson DR. Identification of the transcriptional regulatory sequences of human kallikrein 2 and their use in the construction of calydon virus 764, an attenuated replication competent adenovirus for prostate cancer therapy. Cancer Res 1999; 59(7): 1498–504
DeWeese TL, van der Poel H, Li S, et al. A phase I trial of CV706, a replication-competent, PSA selective oncolytic adenovirus, for the treatment of locally recurrent prostate cancer following radiation therapy. Cancer Res 2001; 61(20): 7464–72
Freytag SO, Rogulski KR, Paielli DL, et al. A novel three-pronged approach to kill cancer cells selectively: concomitant viral, double suicide gene, and radiotherapy. Hum Gene Ther 1998; 9(9): 1323–33
Rogulski KR, Wing MS, Paielli DL, et al. Double suicide gene therapy augments the antitumor activity of a replication-competent lytic adenovirus through enhanced cytotoxicity and radiosensitization. Hum Gene Ther 2000; 11(1): 67–76
Paielli DL, Wing MS, Rogulski KR, et al. Evaluation of the biodistribution, persistence, toxicity, and potential of germ-line transmission of a replication-competent human adenovirus following intraprostatic administration in the mouse. Mol Ther 2000; 1(3): 263–74
Kruyt FA, Curiel DT. Toward a new generation of conditionally replicating adenoviruses: pairing tumor selectivity with maximal oncolysis. Hum Gene Ther 2002; 13(4): 485–95
Brenner M. Reports of adenovector “death” are greatly exaggerated [editorial]. Mol Ther 2000; 1(3): 205
Greenberg DS. Stricter regulation proposed for US gene-therapy trials. Lancet 2000; 355(9219): 1977
Smaglik P. NIH tightens up monitoring of gene-therapy mishaps. Nature 2000; 404(6773): 5
Herman JR, Adler HL, Aguilar-Cordova E, et al. In situ gene therapy for adenocarcinoma of the prostate: a phase I clinical trial. Hum Gene Ther 1999; 10(7): 1239–49
Steiner MS, Anthony CT, Lu Y, et al. Antisense c-myc retroviral vector suppresses established human prostate cancer. Hum Gene Ther 1998; 9(5): 747–55
Logg CR, Tai CK, Logg A, et al. A uniquely stable replication-competent retrovirus vector achieves efficient gene delivery in vitro and in solid tumors. Hum Gene Ther 2001; 12(8): 921–32
Sanda MG, Ayyagari SR, Jaffee EM, et al. Demonstration of a rational strategy for human prostate cancer gene therapy. J Urol 1994; 151(3): 622–8
Simons JW, Mikhak B, Chang JF, et al. Induction of immunity to prostate cancer antigens: results of a clinical trial of vaccination with irradiated autologous prostate tumor cells engineered to secrete granulocyte-macrophage colony-stimulating factor using ex vivo gene transfer. Cancer Res 1999; 59(20): 5160–8
Walker JR, McGeagh KG, Sundaresan P, et al. Local and systemic therapy of human prostate adenocarcinoma with the conditionally replicating herpes simplex virus vector G207. Hum Gene Ther 1999; 10(13): 2237–43
Robbins PD, Ghivizzani SC. Viral vectors for gene therapy. Pharmacol Ther 1998; 80(1): 35–47
Sundaresan P, Hunter WD, Martuza RL, et al. Attenuated, replication-component herpes simplex virus type 1 mutant G207: safety evaluation in mice. J Virol 2000; 74: 3832–41
Mineta T, Rabkin SD, Yazaki T, et al. Attenuated multi-mutatad herpes simplex virus-1 for the treatment of malignant gliomas. Nat Med 1995; 1: 938–43
Hunter WD, Martuza RL, Feigenbaum F, et al. Attenuated, replication-competent herpes simplex virus type 1 mutant G207: safety evaluation of intracerebral injection in nonhuman primates. J Virol 1999; 73: 6319–26
Markert JM, Medlock MD, Rabkin SD, et al. Conditionally replicating herpes simplex virus mutant, G207 for the treatment of malignant glioma: results of a phase I trial. Gene Ther 2000; 7(10): 867–74
Varghese S, Newsome JT, Rabkin SD, et al. Preclinical safety evaluation of G207, a replication-competent herpes simplex virus type 1, inoculated intraprostatically in mice and nonhuman primates. Hum Gene Ther 2001; 12(8): 999–1010
Chase M, Chung RY, Chiocca EA. An oncolytic viral mutant that delivers the CYP2B1 transgene and augments cyclophosphamide chemotherapy. Nat Biotechnol 1998; 16(5): 444–8
Andreansky S, He B, van Cott J, et al. Treatment of intracranial gliomas in immunocompetent mice using herpes simplex viruses that express murine interleukins. Gene Ther 1998; 5(1): 121–30
Advani SJ, Chung SM, Yan SY, et al. Replication-competent, nonneuroinvasive genetically engineered herpes virus is highly effective in the treatment of therapy-resistant experimental human tumors. Cancer Res 1999; 59(9): 2055–8
Mukherjee S, Haenel T, Himbeck R, et al. Replication-restricted vaccinia as a cytokine gene therapy vector in cancer: persistent transgene expression despite antibody generation. Cancer Gene Ther 2000; 7(5): 663–70
von Mehren M, Arlen P, Tsang KY, et al. Pilot study of a dual gene recombinant avipox vaccine containing both carcinoembryonic antigen (CEA) and B7.1 transgenes in patients with recurrent CEA-expressing adenocarcinomas. Clin Cancer Res 2000; 6(6): 2219–28
Eder JP, Kantoff PW, Roper K, et al. A phase I trial of a recombinant vaccinia virus expressing prostate-specific antigen in advanced prostate cancer. Clin Cancer Res 2000; 6(5): 1632–8
Gnant MF, Puhlmann M, Alexander Jr HR, et al. Systemic administration of a recombinant vaccinia virus expressing the cytosine deaminase gene and subsequent treatment with 5-fluorocytosine leads to tumor-specific gene expression and prolongation of survival in mice. Cancer Res 1999; 59(14): 3396–403
Puhlmann M, Brown CK, Gnant M, et al. Vaccinia as a vector for tumor-directed gene therapy: biodistribution of a thymidine kinase-deleted mutant. Cancer Gene Ther 2000; 7(1): 66–73
Kawakita M, Rao GS, Ritchey JK, et al. Effect of canarypox virus (ALVAC)- mediated cytokine expression on murine prostate tumor growth. J Natl Cancer Inst 1997; 89(6): 428–36
Ponnazhagan S, Curiel DT, Shaw DR, et al. Adeno-associated virus for cancer gene therapy. Cancer Res 2001; 61(17): 6313–21
Vieweg J, Boczkowski D, Roberson KM, et al. Efficient gene transfer with adeno-associated virus-based plasmids complexed to cationic liposomes for gene therapy of human prostate cancer. Cancer Res 1995; 55(11): 2366–72
Wilcox ME, Yang W, Senger D, et al. Reovirus as an oncolytic agent against experimental human malignant gliomas. J Natl Cancer Inst 2001; 93(12): 903–12
Hirasawa K, Nishikawa SG, Norman KL, et al. Oncolytic reovirus against ovarian and colon cancer. Cancer Res 2002; 62(6): 1696–701
Norman KL, Coffey MC, Hirasawa K, et al. Reovirus oncolysis of human breast cancer. Hum Gene Ther 2002; 13(5): 641–52
Gioeli D, Mandell JW, Petroni GR, et al. Activation of mitogen-activated protein kinase associated with prostate cancer progression. Cancer Res 1999; 59(2): 279–84
Thompson T, Timme T, Bangma C, et al. Molecular biology of prostate cancer. In: Vogelzang NJSP, Shipley WU, Coffey DS, editors. Comprehensive textbook of genitourinary oncology. Philadelphia (PA): Lippincott Williams and Wilkins, 2000: 553–64
Sirotnak FM, Sepp-Lorenzino L, Kohl NE, et al. A peptidomimetic inhibitor of ras functionality markedly suppresses growth of human prostate tumor xenografts in mice: prospects for long-term clinical utility. Cancer Chemother Pharmacol 2000; 46(1): 79–83
Sun WH, Burkholder JK, Sun J, et al. In vivo cytokine gene transfer by gene gun reduces tumor growth in mice. Proc Natl Acad Sci U S A 1995; 92(7): 2889–93
Rakhmilevich AL, Turner J, Ford MJ, et al. Gene gun-mediated skin transfection with interleukin 12 gene results in regression of established primary and metastatic murine tumors. Proc Natl Acad Sci U S A 1996; 93(13): 6291–6
Huber PE, Pfisterer P. In vitro and in vivo transfection of plasmid DNA in the Dunning prostate tumor R3327-AT1 is enhanced by focused ultrasound. Gene Ther 2000; 7(17): 1516–25
Kashani-Sabet M, Scanion KJ. Application of ribozymes to cancer gene therapy. Cancer Gene Ther 1995; 2(3): 213–23
Belldegrun A, Tso CL, Zisman A, et al. Interleukin 2 gene therapy for prostate cancer: phase I clinical trial and basic biology. Hum Gene Ther 2001; 12(8): 883–92
Xu L, Frederik P, Pirollo KF, et al. Self-assembly of a virus-mimicking nanostructure system for efficient tumor-targeted gene delivery. Hum Gene Ther 2002; 13(3): 469–81
Yu JY, DeRuiter SL, Turner DL. RNA interference by expression of short-interfering RNAs and hairpin RNAs in mammalian cells. Proc Natl Acad Sci U S A 2002; 99(9): 6047–52
Geiger T, Muller M, Monia BP, et al. Antitumor activity of a C-raf antisense oligonucleotide in combination with standard chemotherapeutic agents against various human tumors transplanted subcutaneously into nude mice. Clin Cancer Res 1997; 3(7): 1179–85
Balaji KC, Koul H, Mitra S, et al. Antiproliferative effects of c-myc antisense oligonucleotide in prostate cancer cells: a novel therapy in prostate cancer. Urology 1997; 50(6): 1007–15
Eder IE, Culig Z, Ramoner R, et al. Inhibition of LncaP prostate cancer cells by means of androgen receptor antisense oligonucleotides. Cancer Gene Ther 2000; 7(7): 997–1007
Miyake H, Chi KN, Gleave ME. Antisense TRPM-2 oligodeoxynucleotides chemosensitize human androgen-independent PC-3 prostate cancer cells both in vitro and in vivo. Clin Cancer Res 2000; 6(5): 1655–63
Gleave ME, Miyake H, Zellweger T, et al. Use of antisense oligonucleotides targeting the antiapoptotic gene, clusterin/testosterone-repressed prostate message 2, to enhance androgen sensitivity and chemosensitivity in prostate cancer. Urology 2001; 58(2 Suppl. 1): 39–49
Miyake H, Hara I, Kamidono S, et al. Novel therapeutic strategy for advanced prostate cancer using antisense oligodeoxynucleotides targeting anti-apoptotic genes upregulated after androgen withdrawal to delay androgen-independent progression and enhance chemosensitivity. Int J Urol 2001; 8(7): 337–49
Banerjee D. Genasense (Genta Inc). Curr Opin Investig Drugs 2001; 2(4): 574–80
Benimetskaya L, Miller P, Benimetsky S, et al. Inhibition of potentially antiapoptotic proteins by antisense protein kinase C-alpha (Isis 3521) and antisense bcl-2 (G3139) phosphorothioate oligodeoxynucleotides: relationship to the decreased viability of T24 bladder and PC3 prostate cancer cells. Mol Pharmacol 2001; 60(6): 1296–307
Chi KN, Gleave ME, Klasa R, et al. A phase I dose-finding study of combined treatment with an antisense Bcl-2 oligonucleotide (Genasense) and mitoxan-trone in patients with metastatic hormone-refractory prostate cancer. Clin Cancer Res 2001; 7(12): 3920–7
Morris MJ, Tong WP, Cordon-Cardo C, et al. Phase I trial of BCL-2 antisense oligonucleotide (G3139) administered by continuous intravenous infusion in patients with advanced cancer. Clin Cancer Res 2002; 8(3): 679–83
Eder IE, Hoffmann J, Rogatsch H, et al. Inhibition of LNCaP prostate tumor growth in vivo by an antisense oligonucleotide directed against the human androgen receptor. Cancer Gene Ther 2002; 9(2): 117–25
Yu DC, Chen Y, Seng M, et al. The addition of adenovirus type 5 region E3 enables calydon virus 787 to eliminate distant prostate tumor xenografts. Cancer Res 1999; 59(17): 4200–3
Uchida A, O’Keefe DS, Bacich DJ, et al. In vivo suicide gene therapy model using a newly discovered prostate-specific membrane antigen promoter/enhancer: a potential alternative approach to androgen deprivation therapy. Urology 2001; 58(2 Suppl. 1): 132–9
Nasto B. Questions about systemic adenovirus delivery. Mol Ther 2002; 5: 652–3
Templeton NS, Lasic DD, Frederik PM, et al. Improved DNA: liposome complexes for increased systemic delivery and gene expression. Nat Biotechnol 1997; 15(7): 647–52
Xu L, Pirollo KF, Tang WH, et al. Transferrin-liposome-mediated systemic p53 gene therapy in combination with radiation results in regression of human head and neck cancer xenografts. Hum Gene Ther 1999; 10(18): 2941–52
Pirollo KF, Xu L, Chang EH. Non-viral gene delivery for p53. Curr Opin Mol Ther 2000; 2(2): 168–75
Xu L, Tang WH, Huang CC, et al. Systemic p53 gene therapy of cancer with immunolipoplexes targeted by anti-transferrin receptor scFv. Mol Med 2001; 7(10): 723–34
Templeton NS. Developments in liposomal gene delivery systems. Expert Opin Biol Ther 2001; 1(4): 567–70
Vogelstein B, Kinzler KW. P53 function and dysfunction. Cell 1992; 70(4): 523–6
Thompson T, Timme T, Sehgal I. The role of p53 in prostate cancer progression. In: Fortner J, Sharp P, editors. Accomplishments in cancer research, 1996. Philadelphia: Lippincott-Raven Publishers, 1996: 280–89
Levine AJ. p53, the cellular gatekeeper for growth and division. Cell 1997; 88(3): 323–31
el-Deiry WS. Regulation of p53 downstream genes. Semin Cancer Biol 1998; 8(5): 345–57
Burns TF, El-Deiry WS. The p53 pathway and apoptosis. J Cell Physiol 1999; 181(2): 231–9
Eastham JA, Hall SJ, Sehgal I, et al. In vivo gene therapy with p53 or p21 adenovirus for prostate cancer. Cancer Res 1995; 55(22): 5151–5
Yang C, Cirielli C, Capogrossi MC, et al. Adenovirus-mediated wild-type p53 expression induces apoptosis and suppresses tumorigenesis of prostatic tumor cells. Cancer Res 1995; 55(19): 4210–3
Ko SC, Gotoh A, Thalmann GN, et al. Molecular therapy with recombinant p53 adenovirus in an androgen-independent, metastatic human prostate cancer model. Hum Gene Ther 1996; 7(14): 1683–91
Gotoh A, Kao C, Ko SC, et al. Cytotoxic effects of recombinant adenovirus p53 and cell cycle regulator genes (p21 WAF1/CIP1 and pl6CDKN4) in human prostate cancers. J Urol 1997; 158(2): 636–41
Belldegrun A, Figlin RA. 192-(1997-06) A phase I study in patients with locally advanced or recurrent adenocarcinoma of the prostate using SCH58500 (rAd/ p53) administered by intratumoral injection. Sponsor: Schering-Plough Corporation [online]. Available from URL: http://http://www4.od.nih.gov/oba/rac//clinical-trial.htm [Accessed 2002 May 24]
Logothetis C. 217-(1997-10) A tolerance and efficacy study of intraprostatic INGN 201 followed by pathological staging and possible radical prostatectomy in patients with locally advanced prostate cancer. Sponsor: Introgen Therapeutics, Inc. [online]. Available from URL: http://http://www.od.nih.gov/oba/rac//clinicaltrial.htm [Accessed 2002 May 24]
Pollack A. 418-(2000-10) A randomized phase II study of Ad5CMV-p53 plus radioactive seed implant vs seed implant alone for PSA relapse after external beam radiotherapy for prostate cancer. Sponsor: Introgen Therapeutics, Inc. [online]. Available from URL: http://http://www.od.nih.gov/oba/rac//clinicaltrial.htm [Accessed 2002 May 24]
Merritt JA, Roth JA, Logothetis CJ. Clinical evaluation of adenoviral-mediated p53 gene transfer: review of INGN 201 studies. Semin Oncol 2001; 28(5 Suppl. 16): 105–14
Harper JW. Cyclin dependent kinase inhibitors. Cancer Surv 1997; 29: 91–107
Serrano M, Hannon GJ, Beach D. A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature 1993; 366(6456): 704–7
Jarrard DF, Bova GS, Ewing CM, et al. Deletional, mutational, and methylation analyses of CDKN2 (pl6/MTS1) in primary and metastatic prostate cancer. Genes Chromosomes Cancer 1997; 19(2): 90–6
Steiner MS, Zhang Y, Farooq F, et al. Adenoviral vector containing wild-type pl6 suppresses prostate cancer growth and prolongs survival by inducing cell senescence. Cancer Gene Ther 2000; 7(3): 360–72
Allay JA, Steiner MS, Zhang Y, et al. Adenovirus pl6 gene therapy for prostate cancer. World J Urol 2000; 18(2): 111–20
Gingrich JR. 338-(1999-09) A tolerance and efficacy study of neoadjuvant intraprostatic GTx-001 followed by radical prostatectomy in patients with locally advanced prostate cancer. Sponsor: Genotherapeutics, Inc [online]. Available from URL: http://http://www.od.nih.gOv/oba/rac//clinicaltrial.htm [Accessed 2002 May 24]
Lin SH, Pu YS. Function and therapeutic implication of C-CAM cell-adhesion molecule in prostate cancer. Semin Oncol 1999; 26(2): 227–33
Kleinerman DI, Zhang WW, Lin SH, et al. Application of a tumor suppressor (C-CAM1)-expressing recombinant adenovirus in androgen-independent human prostate cancer therapy: a preclinical study. Cancer Res 1995; 55(13): 2831–6
Lin SH, Pu YS, Luo W, et al. Schedule-dependence of C-CAM1 adenovirus gene therapy in a prostate cancer model. Anticancer Res 1999; 19(1A): 337–40
Steiner MS, Zhang X, Wang Y, et al. Growth inhibition of prostate cancer by an adenovirus expressing a novel tumor suppressor gene, pHyde. Cancer Res 2000; 60(16): 4419–25
McDonnell TJ, Troncoso P, Brisbay SM, et al. Expression of the protooncogene bcl-2 in the prostate and its association with emergence of androgen-independent prostate cancer. Cancer Res 1992; 52(24): 6940–4
Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science 1998; 281(5381): 1322–6
Dorai T, Goluboff ET, Olsson CA, et al. Development of a hammerhead ribozyme against BCL-2: II. ribozyme treatment sensitizes hormone-resistant prostate cancer cells to apoptotic agents. Anticancer Res 1997; 17(5A): 3307–12
Dorai T, Olsson CA, Katz AE, et al. Development of a hammerhead ribozyme against bcl-2:I. preliminary evaluation of a potential gene therapeutic agent for hormone-refractory human prostate cancer. Prostate 1997; 32(4): 246–58
Dorai T, Perlman H, Walsh K, et al. A recombinant defective adenoviral agent expressing anti-bcl-2 ribozyme promotes apoptosis of bcl-2-expressing human prostate cancer cells. Int J Cancer 1999; 82(6): 846–52
Thompson TC, Timme TL, Sehgal I. Oncogenes, growth factors, and hormones in prostate cancer. In: Dickson RB, Salomon DS, editors. Hormones and growth factors in development and neoplasia. New York: Wiley-Liss Inc, 1998: 327–59
Holt JT, Steiner MS. 123-(1995-09) gene therapy for the treatment of advanced prostate cancer by in vivo transduction with prostate-targeted retroviral vectors expressing antisense c-myc RNA [online]. Available from URL: http://http://www.od.nih.gov/oba/rac//clinicaltrial.htm [Accessed 2002 May 24]
Thornberry NA, Lazebnik Y. Caspases: enemies within. Science 1998; 281(5381): 1312–6
Marcelli M, Cunningham GR, Walkup M, et al. Signaling pathway activated during apoptosis of the prostate cancer cell line LNCaP: overexpression of caspase-7 as a new gene therapy strategy for prostate cancer. Cancer Res 1999; 59(2): 382–90
Xie X, Zhao X, Liu Y, et al. Adenovirus-mediated tissue-targeted expression of a caspase-9-based artificial death switch for the treatment of prostate cancer. Cancer Res 2001; 61(18): 6795–804
Shariat SF, Desai S, Song W, et al. Adenovirus-mediated transfer of inducible caspases: a novel “death switch” gene therapeutic approach to prostate cancer. Cancer Res 2001; 61(6): 2562–71
Li X, Marani M, Yu J, et al. Adenovirus-mediated Bax overexpression for the induction of therapeutic apoptosis in prostate cancer. Cancer Res 2001; 61(1): 186–91
Lowe SL, Rubinchik S, Honda T, et al. Prostate-specific expression of Bax delivered by an adenoviral vector induces apoptosis in LNCaP prostate cancer cells. Gene Ther 2001; 8(18): 1363–71
Chung TD, Mauceri HJ, Hallahan DE, et al. Tumor necrosis factor-alpha-based gene therapy enhances radiation cytotoxicity in human prostate cancer. Cancer Gene Ther 1998; 5(6): 344–9
Griffith TS, Anderson RD, Davidson BL, et al. Adenoviral-mediated transfer of the TNF-related apoptosis-inducing ligand/Apo-2 ligand gene induces tumor cell apoptosis. J Immunol 2000; 165(5): 2886–94
Rodriguez R, Lim HY, Bartkowski LM, et al. Identification of diphtheria toxin via screening as a potent cell cycle and p53-independent cytotoxin for human prostate cancer therapeutics. Prostate 1998; 34(4): 259–69
Higuchi H, Bronk SF, Bateman A, et al. Viral fusogenic membrane glycoprotein expression causes syncytia formation with bioenergetic cell death: implications for gene therapy. Cancer Res 2000; 60(22): 6396–402
Bateman A, Bullough F, Murphy S, et al. Fusogenic membrane glycoproteins as a novel class of genes for the local and immune-mediated control of tumor growth. Cancer Res 2000; 60(6): 1492–7
Galanis E, Bateman A, Johnson K, et al. Use of viral fusogenic membrane glycoproteins as novel therapeutic transgenes in gliomas. Hum Gene Ther 2001; 12(7): 811–21
Springer CJ, Niculescu-Duvaz I. Prodrug-activating systems in suicide gene therapy. J Clin Invest 2000; 105(9): 1161–7
Huber BE, Austin EA, Richards CA, et al. Metabolism of 5-fluorocytosine to 5- fluorouracil in human colorectal tumor cells transduced with the cytosine deaminase gene: significant antitumor effects when only a small percentage of tumor cells express cytosine deaminase. Proc Natl Acad Sci USA 1994; 91(17): 8302–6
Bi WL, Parysek LM, Warnick R, et al. In vitro evidence that metabolic cooperation is responsible for the bystander effect observed with HSV tk retroviral gene therapy. Hum Gene Ther 1993; 4(6): 725–31
Barba D, Hardin J, Sadelain M, et al. Development of anti-tumor immunity following thymidine kinase-mediated killing of experimental brain tumors. Proc Natl Acad Sci U S A 1994; 91: 4348–52
Ramesh R, Marrogi AJ, Munshi A, et al. In vivo analysis of the ‘bystander effect’: a cytokine cascade. Exp Hematol 1996; 24(7): 829–38
Melcher A, Todryk S, Hardwick N, et al. Tumor immunogenicity is determined by the mechanism of cell death via induction of heat shock protein expression. Nat Med 1998; 4(5): 581–7
Ram Z, Walbridge S, Shawker T, et al. The effect of thymidine kinase transduction and ganciclovir therapy on tumor vasculature and growth of 9L gliomas in rats. J Neurosurg 1994; 81(2): 256–60
Freytag SO, Paielli D, Wing M, et al. Efficacy and toxicity of replication-component adenovirus-mediated double suicide gene therapy in combination with radiation therapy in an orthotopicmouse prostate cancer model. Int J Radiat Oncol Biol Phys 2002; 54: 873–85
Eastham JA, Chen SH, Sehgal I, et al. Prostate cancer gene therapy: herpes simplex virus thymidine kinase gene transduction followed by ganciclovir in mouse and human prostate cancer models. Hum Gene Ther 1996; 7(4): 515–23
Hall SJ, Mutchnik SE, Chen SH, et al. Adenovirus-mediated herpes simplex virus thymidine kinase gene and ganciclovir therapy leads to systemic activity against spontaneous and induced metastasis in an orthotopic mouse model of prostate cancer. Int J Cancer 1997; 70(2): 183–7
Hall SJ, Mutchnik SE, Yang G, et al. Cooperative therapeutic effects of androgen ablation and adenovirus-mediated herpes simplex virus thymidine kinase gene and ganciclovir therapy in experimental prostate cancer. Cancer Gene Ther 1999; 6(1): 54–63
Cheon J, Kim HK, Moon DG, et al. Adenovirus-mediated suicide-gene therapy using the herpes simplex virus thymidine kinase gene in cell and animal models of human prostate cancer: changes in tumour cell proliferative activity. BJU Int 2000; 85(6): 759–66
Nasu Y, Bangma C, Hull G, et al. Combination gene therapy with adenoviral vector-mediated HSV-tk+GCV and IL-12 in an orthotopic mouse model for prostate cancer. Prostate Cancer Prostatic Dis 2001; 4: 44–55
Chhikara M, Huang H, Vlachaki MT, et al. Enhanced therapeutic effect of HSV- tk+GCV gene therapy and ionizing radiation for prostate cancer. Mol Ther 2001; 3(4): 536–42
Ayala G, Wheeler TM, Shalev M, et al. Cytopathic effect of in situ gene therapy in prostate cancer. Hum Pathol 2000; 31(7): 866–70
Rainov NG, Kramm CM, Banning U, et al. Immune response induced by retrovirus-mediated HSV-tk/GCV pharmacogene therapy in patients with glioblastoma multiforme. Gene Ther 2000; 7(21): 1853–8
Atkinson G, Hall SJ. Prodrug activation gene therapy and external beam irradiation in the treatment of prostate cancer. Urology 1999; 54(6): 1098–104
Timme TL, Hall SJ, Barrios R, et al. Local inflammatory response and vector spread after direct intraprostatic injection of a recombinant adenovirus containing the herpes simplex virus thymidine kinase gene and ganciclovir therapy in mice. Cancer Gene Ther 1998; 5(2): 74–82
Kubo H, Gardner TA, Wada Y, et al. Phase I dose escalation clinical trial of adenovirus vector carrying osteocalcin promoter-driven herpes simplex virus thymidine kinase in localized and metastatic hormone-refractory prostate cancer. Hum Gene Ther 2003; 14: 227–41
Anello R, Cohen S, Atkinson G, et al. Adenovirus mediated cytosine deaminase gene transduction and 5-fluorocytosine therapy sensitizes mouse prostate cancer cells to irradiation. J Urol 2000; 164(6): 2173–7
Parker WB, King SA, Allan PW, et al. In vivo gene therapy of cancer with E. coli purine nucleoside phosphorylase. Hum Gene Ther 1997; 8(14): 1637–44
Martiniello-Wilks R, Tsatralis T, Russell P, et al. Transcription-targeted gene therapy for androgen-independent prostate cancer. Cancer Gene Ther 2002; 9(5): 443–52
Rogulski KR, Kim JH, Kim SH, et al. Glioma cells transduced with an Escherichia coli CD/HSV-1 TK fusion gene exhibit enhanced metabolic suicide and radi-osensitivity. Hum Gene Ther 1997; 8(1): 73–85
Blackburn RV, Galoforo SS, Corry PM, et al. Adenoviral transduction of a cytosine deaminase/thymidine kinase fusion gene into prostate carcinoma cells enhances prodrug and radiation sensitivity. Int J Cancer 1999; 82(2): 293–7
Freytag SO, Khil M, Stricker H, et al. Phase I study of replication-competent adenovirus-mediated double suicide gene therapy for the treatment of locally recurrent prostate cancer. Cancer Res 2002; 62: 4968–76
Freytag SO, Strieker H, Pegg J, et al. Phase I study of replication-competent adenovirus-mediated double-suicide gene therapy in combination with conventional-dose three-dimensional conformai radiation therapy for the treatment of newly diagnosed, intermediate- to high-risk prostate cancer. Cancer Res 2003; 63: 7497–506
Okada T, Shah M, Higginbotham JN, et al. AV.TK-mediated killing of subcutaneous tumors in situ results in effective immunization against established secondary intracranial tumor deposits. Gene Ther 2001; 8(17): 1315–22
Correale P, Walmsley K, Zaremba S, et al. Generation of human cytolytic T lymphocyte lines directed against prostate-specific antigen (PSA) employing a PSA oligoepitope peptide. J Immunol 1998; 161(6): 3186–94
Sanda MG, Smith DC, Charles LG, et al. Recombinant vaccinia-PSA (PROSTVAC) can induce a prostate-specific immune response in androgen-modulated human prostate cancer. Urology 1999; 53(2): 260–6
Meidenbauer N, Harris DT, Spitler LE, et al. Generation of PSA-reactive effector cells after vaccination with a PSA-based vaccine in patients with prostate cancer. Prostate 2000; 43(2): 88–100
Terasawa H, Tsang KY, Gulley J, et al. Identification and characterization of a human agonist cytotoxic T-lymphocyte epitope of human prostate-specific antigen. Clin Cancer Res 2002; 8(1): 41–53
Xu J, Stolk JA, Zhang X, et al. Identification of differentially expressed genes in human prostate cancer using subtraction and microarray. Cancer Res 2000; 60(6): 1677–82
Xu J, Kalos M, Stolk JA, et al. Identification and characterization of prostein, a novel prostate-specific protein. Cancer Res 2001; 61(4): 1563–8
Chen ME, Lin SH, Chung LW, et al. Isolation and characterization of PAGE-1 and GAGE-7: new genes expressed in the LNCaP prostate cancer progression model that share homology with melanoma-associated antigens. J Biol Chem 1998; 273(28): 17618–25
Fong L, Ruegg CL, Brockstedt D, et al. Induction of tissue-specific autoimmune prostatitis with prostatic acid phosphatase immunization: implications for immunotherapy of prostate cancer. J Immunol 1997; 159(7): 3113–7
McNeel DG, Nguyen LD, Disis ML. Identification of T helper epitopes from prostatic acid phosphatase. Cancer Res 2001; 61(13): 5161–7
Zhang S, Zhang HS, Reuter VE, et al. Expression of potential target antigens for immunotherapy on primary and metastatic prostate cancers. Clin Cancer Res 1998; 4(2): 295–302
Dhanasekaran SM, Barrette TR, Ghosh D, et al. Delineation of prognostic biomarkers in prostate cancer. Nature 2001; 412(6849): 822–6
Saffran DC, Reiter RE, Jakobovits A, et al. Target antigens for prostate cancer immunotherapy. Cancer Metastasis Rev 1999; 18(4): 437–49
Chen YT, Scanlan MJ, Sahin U, et al. A testicular antigen aberrantly expressed in human cancers detected by autologous antibody screening. Proc Natl Acad Sci U S A 1997; 94(5): 1914–8
Nakada T, Noguchi Y, Satoh S, et al. NY-ESO-1 mRNA espression andimmu-nogenicity in advanced prostate cancer. Cancer Immun 2003; 3: 10
Murphy GP, Tjoa BA, Simmons SJ, et al. Infusion of dendritic cells pulsed with HLA-A2-specific prostate-specific membrane antigen peptides: a phase II prostate cancer vaccine trial involving patients with hormone-refractory metastatic disease. Prostate 1999; 38(1): 73–8
Burch PA, Breen JK, Buckner JC, et al. Priming tissue-specific cellular immunity in a phase I trial of autologous dendritic cells for prostate cancer. Clin Cancer Res 2000; 6(6): 2175–82
Small EJ, Fratesi P, Reese DM, et al. Immunotherapy of hormone-refractory prostate cancer with antigen-loaded dendritic cells. J Clin Oncol 2000; 18(23): 3894–903
Heiser A, Maurice MA, Yancey DR, et al. Induction of polyclonal prostate cancer-specific CTL using dendritic cells transfected with amplified tumor RNA. J Immunol 2001; 166(5): 2953–60
Fong L, Brockstedt D, Benike C, et al. Dendritic cell-based xenoantigen vaccination for prostate cancer immunotherapy. J Immunol 2001; 167(12): 7150–6
Correale P, Micheli L, Vecchio MT, et al. A parathyroid-hormone-related-protein (PTH-rP)-specific cytotoxic T cell response induced by in vitro stimulation of tumour-infiltrating lymphocytes derived from prostate cancer metastases, with epitope peptide-loaded autologous dendritic cells and low-dose IL-2. Br J Cancer 2001; 85(11): 1722–30
Heiser A, Coleman D, Dannull J, et al. Autologous dendritic cells transfected with prostate-specific antigen RNA stimulate CTL responses against metastatic prostate tumors. J Clin Invest 2002; 109(3): 409–17
Curiel TJ, Curiel DT. Tumor immunotherapy: inching toward the finish line. J Clin Invest 2002; 109(3): 311–2
Leonard JP, Sherman ML, Fisher GL, et al. Effects of single-dose interleukin-12 exposure on interleukin-12-associated toxicity and interferon- gamma production. Blood 1997; 90(7): 2541–8
Kang WK, Park C, Yoon HL, et al. Interleukin 12 gene therapy of cancer by peritumoral injection of transduced autologous fibroblasts: outcome of a phase I study. Hum Gene Ther 2001; 12(6): 671–84
Nasu Y, Bangma CH, Hull GW, et al. Adenovirus-mediated interleukin-12 gene therapy for prostate cancer: suppression of orthotopic tumor growth and pre-established lung metastases in an orthotopic model. Gene Ther 1999; 6(3): 338–49
Sanford MA, Yan Y, Canfield SE, et al. Independent contributions of GR-1+ leukocytes and Fas/FasL interactions to induce apoptosis following interleukin-12 gene therapy in a metastatic model of prostate cancer. Hum Gene Ther 2001; 12(12): 1485–98
Miles BJ. 449-(2001-01) Phase I study of adenoviral vector delivery of the IL-12 gene in men with local recurrence of prostate cancer after irradiation failure [online]. Available from URL: http://http://www.od.nih.gov/oba/rac//clinicaltrial.htm [Accessed 2002 May 24]
Suzuki K, Nakazato H, Matsui H, et al. NK cell-mediated anti-tumor immune response to human prostate cancer cell, PC-3: immunogene therapy using a highly secretable form of interleukin-15 gene transfer. J Leukoc Biol 2001; 69(4): 531–7
Chen L, Ashe S, Brady WA, et al. Costimulation of antitumor immunity by the B7 counterreceptor for the T lymphocyte molecules CD28 and CTLA-4. Cell 1992; 71(7): 1093–102
Hull GW, McCurdy MA, Nasu Y, et al. Prostate cancer gene therapy: comparison of adenovirus-mediated expression of interleukin 12 with interleukin 12 plus B7-1 for in situ gene therapy and gene-modified, cell-based vaccines. Clin Cancer Res 2000; 6(10): 4101–9
Acknowledgements
The gene therapy research from our laboratory was supported by the National Cancer Institute (CA50588, CA68814 and SPORE P50-58204), CaPCURE, and the General Clinical Research Center at The Methodist Hospital. We appreciate the helpful comments of Dr Svend O. Freytag of the Henry Ford Health Care System.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gdor, Y., Timme, T.L., Kadmon, D. et al. Strategies for Prostate Cancer Gene Therapy. Am J Cancer 3, 79–95 (2004). https://doi.org/10.2165/00024669-200403020-00002
Published:
Issue Date:
DOI: https://doi.org/10.2165/00024669-200403020-00002