Abstract
▴ Docetaxel and cisplatin are well established antineoplastic agents with activity against NSCLC. The combination exhibited additive cytotoxic activity against human NSCLC cell lines in vitro.
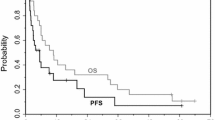
▴ In a large phase III trial in chemotherapy-naive patients with advanced NSCLC, survival with docetaxel plus cisplatin was statistically noninferior to that with the control regimen of vinorelbine plus cisplatin. Overall response rate with docetaxelplus cisplatin was significantly higher than with the control.
▴ Median survival times, tumor response rates, and median time to progression for patients receiving docetaxel plus cisplatin were similar to those for patients receiving paclitaxel plus cisplatin in another large phase III trial.
▴ Neutropenia was the most common grade 3/4 adverse event in docetaxel/cisplatin recipients (≥69% of patients in the two large phase III trials); these proportions were not significantly different from those for patients receiving controls. Grade 3/4 vomiting, nausea, or anemia were significantly less common than with vinorelbine plus cisplatin, whereas hypersensitivity reactions were significantly more common than with paclitaxel plus cisplatin.

Table. Features and properties of docetaxel (Taxotere®) plus cisplatin (Platinol®)


Similar content being viewed by others

Notes
Use of a tradename is for product identification purposes only, and does not imply endorsement.
References
Jemal A, Thomas A, Murray T, et al. Cancer statistics, 2002. CA Cancer J Clin 2002; 52: 23–47
Simon GR, Bunn PA Jr. Taxanes in the treatment of advanced (stage III and IV) non-small cell lung cancer (NSCLC): recent developments. Cancer Invest 2003; 21(1): 87–104
Socinski MA, Morris DE, Masters GA, et al. Chemotherapeutic management of stage IV non-small cell lung cancer. Chest 2003 Jan; 123(1 Suppl.): 226S–43S
American Society of Clincal Oncology. Clinical practice guidelines: unresectable non-small-cell lung cancer. J Clin Oncol 1997; 15(8): 2996–3018
Cancer Care Ontario. Chemotherapy in stage IV (metastatic) non-small cell lung cancer (practice guideline #7-2) [online]. Available from URL: http://www.cancercare.on.ca [Accessed 2003 Jul 5]
National Comprehensive Cancer Network. Clinical practice guidelines in oncology: non-small call lung cancer, version 1 [online]. Available from URL: http://www.nccn.org [Accessed 2003 Jul 5]
National Cancer Institute. Non-small cell lung cancer (PDQ®): treatment [online]. Available from URL: http://www.nci.nih.gov [Accessed 2003 Jul 5]
Comer AM, Goa KL. Docetaxel: a review of its use in non-small cell lung cancer. Drugs Aging 2000 Jul; 17(1): 53–80
O’Dwyer PJ, Johnson SW, Hamilton TC. Cisplatin and its analogues. In: DeVita VT, Hellman S, Rosenberg SA, editors. Cancer: principles and practice of oncology. 5th ed. Philadelphia (PA): Lippincott-Raven, 1997: 418–32
Aoe K, Kiura K, Ueoka H, et al. Effect of docetaxel with cisplatin or vinorelbine on lung cancer cell lines. Anticancer Res 1999; 19: 291–300
Millward MJ, Zalcberg J, Bishop JE, et al. Phase I trial of docetaxel and cisplatin in previously untreated patients with advanced non-small-cell lung cancer. J Clin Oncol 1997 Feb; 15(2): 750–8
Bruno R, Vivier N, Veyrat-Follet C, et al. Population pharmacokinetics and pharmacokinetic-pharmacodynamic relationships for docetaxel. Invest New Drugs 2001 May; 19(2): 163–9
Clarke SJ, Rivory LT. Clinical pharmacokinetics of docetaxel. Clin Pharmacokinet 1999; 36(2): 99–114
Aventis Pharmaceuticals Inc. Taxotere (docetaxel) injection concentrate — prescribing information (US) [online]. Available from URL: http://www.aventisus.com [Accessed 2003 Jul 5]
Fossella F, Pereira JR, von Pawel J. Randomized, multinational, phase III study of docetaxel plus platinum combinations versus vinorelbine plus cisplatin for advanced non-small-cell lung cancer: the TAX 326 study group. J Clin Oncol 2003; 21(16): 3016–24
Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 2002 Jan; 346(2): 92–8
Kubota K, Watanabe K, Kunitoh H, et al. Final results of a randomized phase III trial of docetaxel and cisplatin versus vindesine and cisplatin in stage IV nonsmall cell lung cancer (NSCLC) [abstract no. 1180]. Proc Am Soc Clin Oncol 2002; 21: 296a. Plus oral presentation at the 38th Annual Meeting of the Am Soc of Clin Oncol; 2002 May 18–21; Orlando
Georgoulias V, Ardavanis A, Agelidou M, et al. Preliminary analysis of a multicenter phase III trial comparing docetaxel (D) versus docetaxel/cisplatin (DC) in patients with inoperable advanced and metastatic non-small cell lung cancer (NSCLC) [abstract no. 1163]. Proc Am Soc Clin Oncol 2002; 21: 291a
Final results of phase II randomized study of taxotere (TXT)+ cisplatin (CDDP) versus vinorelbine (V) + CDDP in patients with stage IV non-small cell lung cancer (NSCLC) [abstract no. 1296]. Proc Am Soc Clin Oncol 2002; 21 Pt 1: 325a
Georgoulias V, Samonis G, Papadakis E, et al. Comparison of docetaxel/cisplatin to docetaxel/gemcitabine as first-line treatment of advanced non-small cell lung cancer: early results of a randomized trial. Lung Cancer 2001 Dec; 34Suppl. 4: S47–51
Georgoulias V, Papadakis E, Alexopoulos A, et al. Platinum-based and nonplatinum-based chemotherapy in advanced non-small-cell lung cancer: a randomised multicentre trial. Lancet 2001 May 12; 357: 1478–84
Okamoto H, Watanabe K, Segawa Y, et al. Phase II study of docetaxel and cisplatin in patients with previously untreated metastatic non-small-cell lung cancer. Int J Clin Oncol 2000 Oct; 5: 316–22
Georgoulias V, Androulakis N, Dimopoulos AM, et al. First-line treatment of advanced non-small-cell lung cancer with docetaxel and cisplatin: a multicenter phase II study. Ann Oncol 1998 Mar; 9(3): 331–4
Belani CP, Bonomi P, Dobbs TW, et al. Docetaxel and cisplatin in patients with advanced non-small-cell lung cancer (NSCLC): a multicenter phase II trial. Clinical Lung Cancer 1999; 1(2): 144–50
Kim YH, Kim JS, Choi YH, et al. Phase II study of docetaxel and cisplatin combination chemotherapy in metastatic or unresectable localized non-small-cell lung cancer. Int J Clin Oncol 2002 Apr; 7(2): 114–9
Ho JCM, Tan EH, Wang CH, et al. Preliminary results of a multi-centre Asian trial of docetaxel and cisplatin in advanced non-small cell lung cancer [abstract no. 2702]. Proc Am Soc Clin Oncol 2001; 20 Pt 2: 238b
Amenedo M, Firvida JL, Gonzalez-Quintas A, et al. Docetaxel in combination with fractionated cisplatin as first line treatment of locally advanced or metastatic non-small-cell lung cancer (NSCLC) [abstract no. 2761]. Proc Am Soc Clin Oncol 2003; 22: 687
Faderl B, von Pawel J, Wagner H, et al. Phase II study of docetaxel and cisplatin in a circadian timing as first line chemotherapy (CT) in advanced non small cell lung cancer (NSCLC) [abstract no. 1017]. Eur J Cancer 1999 Sep; 35Suppl. 4: S256
Zalcberg J, Millward M, Bishop J, et al. Phase II study of docetaxel and cisplatin in advanced non-small-cell lung cancer. J Clin Oncol 1998; 16: 1948–53
Le Chevalier T, Monnier A, Douillard JY, et al. Docetaxel (taxotere) plus cisplatin: an active and well-tolerated combination in patients with advanced non-small cell lung cancer. Eur J Cancer 1998; 34(13): 2032–6
Aventis Pharmaceuticals Inc. Media Release; FDA approves avenus’ taxotere for first-line treatment of patients with non-small cell lung cancer [online]. Available from URL: http://www.aventis.com[Accessed 2003 Jul 20]
Fossella FV, Belani CPftT3SG. Phase III study (TAX 326) of docetaxel-cisplatin (DC) and docetaxel-carboplatin (DCb) versus vinorelbine-cisplatin (VC) for the first-line treatment of advanced/metastatic non-small-cell lung cancer (NSCLC): analysis in elderly patients [abstract no. 2528]. Proc Am Soc Clin Oncol 2003; 22: 629. Plus poster presented at the 39th Annual Meeting of the Am Soc of Clin Oncol; 2003 May 31; Chicago. Plus poster presented at the 39th Annual Meeting of the Am Soc of Clin Oncol 2003; Jun 3; Chicago
Langer CJ, Vangel M, Schiller J, et al. Age-specific subanalysis of ECOG 1594: fit elderly patients (70–80 yrs) with NSCLC do as well as younger patients [abstract no. 2571]. Proc Am Soc Clin Oncol 2003; 22: 639
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dando, T.M., Goa, K.L. Docetaxel Plus Cisplatin. Am J Cancer 2, 349–356 (2003). https://doi.org/10.2165/00024669-200302050-00005
Published:
Issue Date:
DOI: https://doi.org/10.2165/00024669-200302050-00005