Abstract
Trastuzumab is a humanized monoclonal antibody developed to target the HER2 receptor which is over-expressed by some cancer cells, including 25 to 30% of breast cancers. Binding with high affinity to the extracellular domain of HER2, trastuzumab inhibits the proliferation of tumor cells that overexpress HER2.
A large well designed multicenter study found that the addition of trastuzumab to either an anthracycline plus cyclophosphamide or to paclitaxel, as first-line therapy for metastatic breast cancer overexpressing the HER2 receptor, significantly increased time to disease progression, rate of objective response, duration of response and survival compared with chemotherapy alone.
Single-agent trastuzumab was associated with an objective response in 15% of extensively pretreated patients with metastatic breast cancer overexpressing HER2, and 26% of previously untreated patients. Patients with a HER2 overexpression level of 3+ using immunohistochemical assay, or a positive HER2 result using fluorescence in situ hybridisation, benefit more from trastuzumab therapy than those with tumors overexpressing at a level of 2+.
Trastuzumab has demonstrated synergistic action with several chemotherapy agents preclinically but the optimal combination clinically is yet to be determined.
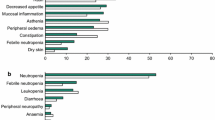
Trastuzumab is generally well tolerated by most patients; the most significant adverse effects being acute fever and/or chills and the potential to cause cardiac dysfunction. Serious adverse events, including anaphylaxis and death, have occurred in 0.25% of patients.
Symptomatic or asymptomatic cardiac dysfunction occurred in 27% of patients receiving an anthracycline and cyclophosphamide combined with trastuzumab. Thus, combination therapy with anthracyclines is not recommended. Symptomatic or asymptomatic cardiac dysfunction occurred in 13% of patients receiving trastuzumab plus paclitaxel and in 4.7% of patients receiving trastuzumab alone.
In conclusion, intravenous trastuzumab is effective as a single-agent, and in combination with chemotherapy it significantly improves the median time to disease progression and survival time in patients with metastatic breast cancer overexpressing the HER2 receptor compared with chemotherapy alone. Cardiotoxicity is the main concern with therapy; particularly in patients with pre-existing cardiac dysfunction, the elderly and in combination with, or following, anthracyclines. Trastuzumab is indicated for use with paclitaxel as first-line therapy or as a single agent in second- or third-line treatment regimens for patients with metastatic breast cancer overexpressing HER2. Investigation is ongoing to ascertain the optimal combination regimen containing trastuzumab and antineoplastic agents. In addition, current research is focusing on the optimal timing, sequencing and duration of therapy as well as administration in the neoadjuvant and adjuvant setting.
Similar content being viewed by others
References
Albaneil J, Baselga J. Trastuzumab, a humanized anti-HER2 monoclonal antibody, for the treatment of breast cancer. Drugs Today 1999; 35(12): 931–46
Press MF, Bernstein L, Thomas PA, et al. HER-2/neu gene amplification characterized by fluorescence in situ hybridization: poor prognosis in node-negative breast carcinomas. J Clin Oncol 1997 Aug; 15(8): 2894–904
Carter P, Presta L, Gorman CM, et al. Humanization of an anti-p185HER2 antibody for human cancer therapy. Proc Natl Acad Sci USA 1992 May; 89 (Immunology): 4285–9
Eisenhauer EA. From the molecule to the clinic — inhibiting HER2 to treat breast cancer [editorial]. N Engl J Med 2001 Mar 15; 344(11): 841–2
Baselga J, Norton L, Albanell J, et al. Recombinant humanized anti-HER2 antibody (Herceptin®) enhances the antitumor activity of paclitaxel and doxorubicin against HER2/neu overexpressing human breast cancer xenografts. Cancer Res 1998 Jul 1; 58: 2825–31
Pegram M, Hsu S, Lewis G, et al. Inhibitory effects of combinations of HER-2/neu antibody and chemotherapeutic agents used for treatment of human breast cancers. Oncogene 1999 Apr 1; 18: 2241–51
Roche Products Ltd. Herceptin®: Summary of Product Characteristics. 2001 (Data on file)
Pestalozzi BC, Brignoli S. Herceptin® (trastuzumab) in cerebrospinal fluid. Eur J Cancer 2000 Sep; 36 Suppl. 5: 54
Genentech Inc. Herceptin® (trastuzumab): full prescribing information. 2000 Sep
Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001 Mar 15; 344: 783–92
Fyfe GA, Mass R, Murphy M, et al. Survival benefit of trastuzumab (Herceptin) and chemotherapy in older (age>60) patients [abstract no. 189]. Proceedings of the 37th Annual Meeting of the American Society of Clinical Oncology; 2001 May 12–15; San Francisco, 48
KrasnaL, Janku F, Petruzelka L, et al. Herceptin (H) and Taxol(T) in the treatment of women with HER-2/neu overexpressing metastatic breast cancer (MBC): prospective study [abstract no. 2006]. Proc Am Soc Clin Oncol 2001 May 12; 20(2): 64
Krzemieniecki K, Pawlicki M. Herceptin and Taxol combination for metastatic breast cancer in women with HER-2/neu overexpression [abstract]. 11th International Congress on Anti-Cancer Treatment; 2001 Feb 6; Paris, 276
Moreau L, Mouret-Reynier MA, Penault-Llorca F, et al. Addition of herceptin to paclitaxel for Her2 over-expressing advanced breast cancer. Best results in skin metastasis [abstract no. P114]. 11th International Congress on Anti-Cancer Treatment; 2001 Feb 6; Paris, 220
Scholz U, Lück HJ, Schippert C, et al. Trastuzumab (Herceptin) combined with weekly paclitaxel in the treatment of metastatic breast cancer: a phase II study [abstract]. Breast Cancer Res Treat 2000 Nov; 64: 122
Seidman AD, Former MN, Esteva FJ, et al. Weekly trastuzumab and paclitaxel therapy for metastatic breast cancer with analysis of efficacy by HER2 immunophenotype and gene amplification. J Clin Oncol 2001 May; 19(10): 2587–95
Tsavdaridis D, Timotheadou E, Christodoulou C, et al. Weekly administration of paclitaxel, as first line chemotherapy, and Herceptin (transtuzumab) in advanced breast cancer [abstract]. Ann Oncol 2000; 11 Suppl. 4: 32
Verma S, Leyland-Jones B, Ayoub J-P, et al. Efficacy and safety of three weekly herceptin with paclitaxel in women with Her2-positive metastatic breast cancer: preliminary results of a phase II trial [abstract no. 538]. Eur J Cancer 2001 Oct 21; 37 Suppl. 6: S146
Yeung K, Gupta R, Haidak D, et al. Weekly (W) Herceptinr (H, Traztuximab) and one hour Taxol (T, paclitaxel) infusion (WHT) regimen for human epidermal growth factor receptor-2 (HER2) overexpressed (+) metastatic breast cancer (MBC) [abstract]. 36th Proc Am Soc Clin Oncol 2000 May 20; 19: 142a
Burris III HA. Docetaxel (Taxotere) plus trastuzumab (Herceptin) in breast cancer. Semin Oncol 2001 Feb; 28(1) Suppl. 3: 38–44
Esteva FJ, Valero V, Booser D, et al. Phase II study of weekly docetaxel (Taxotere; txt) and trastuzumab (Herceptin; H) for patients with HER-2 overexpressing (HER2+) metastatic breast cancer (MBC) [abstract no. 706]. Eur J Cancer 2001 Oct 21; 37Suppl. 6: S193
Kuzur ME, Albain KS, Huntington SF, et al. A phase II trial of docetaxel and Herceptin in metastatic breast cancer patients overexpressing HER-2 [abstract no. 512]. Proc Am Soc Clin Oncol 2000 May 20; 19: 131a
Meden H, Beneke A, Hesse T, et al. Weekly intravenous recombinant humanized anti-P185HER2 monoclonal antibody (herceptin) plus docetaxel in patients with metastatic breast cancer: a pilot study. Anticancer Res 2001; 21: 1301–6
Uber KA, Nicholson BP, Thor AD, et al. A phase II trial of weekly docetaxel (D) and Herceptin (H) as first- or second-line treatment in HER2 over-expressing metastatic breast cancer [abstract no. 1949]. Proc Am Soc Clin Oncol 2001 May 12; 20(2): 50
Pegram MD, Lipton A, Hayes DF, et al. Phase II study of receptor-enhanced chemosensitivity using recombinant humanized anti-p185HER2neu monoclonal antibody plus cisplatin in patients with HER2/neu-overexpressing metastatic breast cancer refractory to chemotherapy treatment. J Clin Oncol 1998 Aug; 16(8): 2659–71
Bangemann N, Kuhle A, Ebert A, et al. Capecitabine combined with trastuzumab in the therapy of intensively pretreated HER2-overexpressing metastatic breast cancer (MBC) [abstract]. Ann Oncol 2000; 11Suppl. 4: 143
Burstein HJ, Kuter I, Campos SM, et al. Clinical activity of trastuzumab and vinorelbine in women with HER2-overexpressing metastatic breast cancer. J Clin Oncol 2001 May 15; 19(10): 2722–30
Jahanzeb M, Mortimer J, Yunus D, et al. A phase II multicenter trial of weekly herceptin with navelbine in chemonaive patients with her2 positive metastatic breast cancer [abstract no. 710]. Eur J Cancer 2001 Oct 21; 37Suppl. 6: 194
Burris III HA, Hainsworth JD, Miranda FT, et al. Phase II trial of Herceptin induction followed by combination therapy with paclitaxel and carboplatin: a Minnie Pearl Research Network trial [abstract]. Breast Cancer Res Treat 2000; 64: 31
Miller KD, Sisk J, Ansari R, et al. Gemcitabine, paclitaxel, and trastuzumab in metastatic breast cancer. Oncology 2001 Feb; 15(2 Suppl. 3): 38–40
Bangemann N, Kuhle A, Willrodt RG, et al. Treatment of HER2 overexpressing metastatic breast cancer with trastuzumab (Herceptin®) and chemotherapy [abstract]. Breast Cancer Res Treat 2000 Nov; 64: 123
Nabholtz JM, Pienkowski T, Nothfelt D, et al. Results of two open label multicentre phase II pilot studies with Herceptin in combination with docetaxel and platinum salts (Cis or Carboplatin) (TCH) as therapy for advanced breast cancer (ABC) in women with tumors over-expressing the HER2-neu proto-oncogene [abstract no. 695]. Eur J Cancer 2001 Oct; 37Suppl. 6: 190
Pienkowski T, Fumoleau P, Eiermann J, et al. Taxotere, Cisplatin and Herceptin (TCH) in first-line HER2 positive metastatic breast cancer (MBC) patients, a phase II pilot study by the breast cancer international research group (BCIRG 101) [abstract]. Proc Am Soc Clin Oncol 2001; 20(2): A2030
Cobleigh MA, Vogel CL, Tripathy D, et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol 1999 Sep; 17: 2639–48
Vogel CL, Cobleigh MA, Tripathy D, et al. First-line herceptin® monotherapy in metastatic breast cancer. Oncology 2001; 61Suppl. 2: 37–42
Tubbs RR, Pettay JD, Roche PC, et al. Discrepancies in clinical laboratory testing of eligibility for trastuzumab therapy: apparent immunohistochemical false-positives do not get the message. J Clin Oncol 2001 May; 19(10): 2714–21
Acosta G, Askaa J, Bilous M, et al. Initial report of the HER2000 study: a multinational study of HER2 status of breast cancer using immunohistochemistry (IHC; Herceptest) [abstract]. Eur J Cancer 2001 Oct 21; 37Suppl. 6: S241
Osoba D, Slamon DJ, Burchmore M, et al. Effects of treatment with Her2mab (trastuzumab/Herceptin) plus chemotherapy (H+C) versus chemotherapy alone (C) on health-related quality of life (HRQL) in women with HER-2/neu-overexpressing metastatic breast cancer [abstract no. 109]. Proc Am Soc Clin Oncol 2001 May 12; 20 (Pt 1): 28
Osoba D, Burchmore MJ, Ash M, et al. Health related quality of life (HRQL) of patients treated with first-line, single agent, trastuzumab (Herceptin) for metastatic Her-2 overexpressing breast cancer (MBC) [abstract]. 36th Proc Am Soc Clin Oncol 2000 May 20; 19: 436a
Lieberman G, Burchmore MJ, Ferhenbacher L, et al. Health related quality of life (HRQL) of patients with HER-2 overexpressing, metastatic breast cancer (MBC) treated with Herceptin (trastuzumab) as a single agent [abstract no. 1613]. Proc Am Soc Clin Oncol 1999 May 15; 18: 417a
Keefe DL, Spaltro B, Pierri MK. Cardiovascular adverse effects of trastuzumab in patients with stage IV breast cancer [abstract]. Clin Pharmacol Ther 2001 Feb; 69 Suppl.: 6
Genentech Inc. Important drug warning: May 3, 2000 [letter]. US Food and Drug Administration website. Available from URL: http://www.fda.gov/medwatch/safety/2000/hercep.htm [Accessed 2002 Jun 21]
EMEA. EMEA public statement on trastuzumab (Herceptin®) — new pharmacokineticdata. 13 Jun 2001
Acknowledgements
The full text of this article appeared in Drugs 2002; 62 (1): 209-243, and was reviewed by: N. Bangemann, Medical Center Benjamin Franklin, Free University, Berlin, Germany; H. Burstein, Dana-Faber Cancer Institute, Boston, Massachussetts, USA; L. A. Carey, UNC Lineberger Comprehensive Cancer Center, University of North Carolina, Chapel Hill, North Carolina, USA; I. O. Ellis, Department of Histopathology, City Hospital, Nottingham, England; V. Harvey, Department of Clinical Oncology, Auckland Hospital, Auckland, New Zealand; J. Horton, H. Lee Moffitt Cancer Center & Research Institute, Tampa, Florida, USA; B. Leyland-Jones, Department of Oncology, McGill University, Montreal, Canada; R. Seshadri, Flinders Medical Centre, Bedford Park, South Australia, Australia.
Author information
Authors and Affiliations
Corresponding author
Additional information
The full text of this article was published in Drugs 2002; 62 (1): 209–243.
Rights and permissions
About this article
Cite this article
McKeage, K., Perry, C.M. Spotlight on Trastuzumab in Metastatic Breast Cancer Overexpressing HER2. Am J Cancer 1, 217–221 (2002). https://doi.org/10.2165/00024669-200201030-00006
Published:
Issue Date:
DOI: https://doi.org/10.2165/00024669-200201030-00006