Abstract
-
▴ The mechanism of action of interferon-β-1b in multiple sclerosis (MS) is not clearly understood, but is thought to involve immunoregulatory activities, including enhancing the suppressor activity of peripheral blood mononuclear cells.
-
▴ In the planned 3-year analysis of the BENEFIT study in patients with a single clinical event suggestive of MS, the relative risk of clinically definite (CD) MS was reduced by 41% in those receiving interferon-β-1b 250 μg every other day for 3 years (early-treatment group) compared with patients who were initially randomized to placebo then switched to interferon-β-1b 250 μg every other day at the end of 2 years or at the onset of CDMS (delayed-treatment group) [p < 0.01].
-
▴ The relative risk of confirmed progression of the expanded disability status scale (EDSS) was reduced by 40% in the early-treatment group compared with the delayed-treatment group over 3 years (p < 0.05).
-
▴ At the end of the 2-year, randomized, placebo-controlled period of the BENEFIT study, the risk of developing CDMS (p < 0.0001) and McDonald-defined MS (p < 0.00001) was significantly lower in the interferon-β-1b group than in the placebo group, and in the magnetic resonance imaging analysis, fewer newly active lesions developed in the interferon-β-1b group (p < 0.001).
-
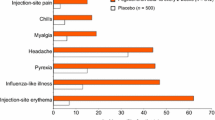
▴ Interferon-β-1b was generally well tolerated. In the 3-year BENEFIT study, neutralizing activity, which was reported in about one-third of the early-treatment group, had no effect on outcome.


Similar content being viewed by others
Notes
The use of trade names is for identification purposes only and does not imply endorsement.
References
O’Connor P. Key issues in the diagnosis and treatment of multiple sclerosis: an overview. Neurology 2002; 59Suppl. 3: Sl–33
The National Collaborating Centre for Chronic Conditions. Multiple sclerosis: national clinical guidance for diagnosis and management in primary and secondary care [online]. Available from URL: http://www.rcplondon.ac.uk/pubs/books/MS/MSfulldocument.pdf [Accessed 2008 Jun 10]
Flachenecker P, Rieckmann P. Early intervention in multiple sclerosis: better outcomes for patients and society? Drugs 2003; 63(15): 1525–33
Stuve O, Bennett JL, Hemmer B, et al. Pharmacological treatment of early multiple sclerosis. Drugs 2008; 68(1): 73–83
Electronic Medicines Compendium. Betaferon® 250 μg/mL: summary of product characteristics [online]. Available from URL: http://emc.medicines.org.Uk/emc/assets/c/html [Accessed 2008 Jun 10]
McCormack PL, Scott LJ. Interferon-β-1b: a review of its use in relapsing-remitting and secondary progressive multiple sclerosis. CNS Drugs 2004; 18(8): 521–46
Kappos L, Freedman MS, Polman CH, et al. Effect of early versus delayed interferon beta-1b treatment on disability after a first clinical event suggestive of multiple sclerosis: a 3-year follow-up analysis of the BENEFIT study. Lancet 2007 Aug 4; 370(9585): 389–97
Betaseron® (interferon beta-1b) for sc injection: US prescribing information. Montville (NJ): Bayer HealthCare Pharmaceuticals Inc, 2007
Karabudak R, Kurne A, Guc D, et al. Effect of interferon β-1a on serum matrix metalloproteinase-9 (MMP-9) and tissue inhibitor of matrix metaaoproteinase (TIMP-1) in relapsing remitting multiple sclerosis patients: one year follow up results. J Neurol 2004: 251: 279–83
Kappos L, Polman CH, Freedman MS, et al. Treatment with interferon beta-1b delays conversion to clinically definite and McDonald MS in patients with clinically isolated syndromes. Neurology 2006; 67: 1242–9
Chiang J, Gloff CA, Yoshizawa CN, et al. Pharmacokinetics of recombinant human interferon-βser in healthy volunteers and its effect on serum neopterin. Pharm Res 1993 Apr; 10(4): 567–72
Poser CM, Paty DW, Scheinberg L, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol 1983; 13(3): 227–31
McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the international panel on the diagnosis of multiple sclerosis. Ann Neurol 2001; 50(1): 121–7
Cutter GR, Baier ML, Rudick RA, et al. Development of a multiple sclerosis functional composite as a clinical trial outcome measure. Brain 1999; 122: 871–82
Acknowledgements and Disclosures
The manuscript was reviewed by: M.S. Freedman, Department of Medicine (Neurology), University of Ottawa, Ottawa, Ontario, Canada; O. Stuve, VA North Texas Health Care System, Neurology Section, Medical Service, Dallas, Texas, USA.
The preparation of this review was not supported by any external funding. During the peer review process, the manufacturer of the agent under review was offered an opportunity to comment on this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
McKeage, K. Interferon-β-1b. CNS Drugs 22, 787–792 (2008). https://doi.org/10.2165/00023210-200822090-00005
Published:
Issue Date:
DOI: https://doi.org/10.2165/00023210-200822090-00005