Summary
Abstract
Quetiapine (Seroquel®), a dibenzothiazepine derivative, is an atypical antipsychotic with demonstrated efficacy in acute schizophrenia. In short-term, randomised, double-blind trials, it was usually more effective than placebo, and was generally effective against both positive and negative symptoms.
Overall, quetiapine (up to 750 mg/day) was at least as effective as chlorpromazine (up to 750 mg/day) and had similar efficacy to haloperidol (up to 16 mg/day) in patients with acute schizophrenia in randomised, double-blind trials; it was at least as effective as haloperidol 20 mg/day in patients with schizophrenia unresponsive or partially responsive to previous antipsychotic treatment. Improvements in overall psychopathology and positive and negative symptoms with quetiapine (up to 800 mg/day) were similar to those with risperidone (up to 8 mg/ day) or olanzapine (15 mg/day) [interim analysis].
Efficacy was maintained for at least 52 weeks in open-label follow-up studies in adult and elderly patients. Quetiapine improved cognitive function versus haloperidol, and depressive symptoms and hostility/aggression versus placebo.
Quetiapine is well tolerated. It is associated with placebo-level incidence of extrapyramidal symptoms (EPS) across its entire dose range, appears to have a low risk for EPS in vulnerable patient groups (e.g. the elderly, adolescents or patients with organic brain disorders) and has a more favourable EPS profile than risperidone. Irrespective of dose, quetiapine, unlike risperidone and amisulpride, does not elevate plasma prolactin levels compared with placebo, and previously elevated levels may even normalise. Quetiapine appears to have minimal short-term effects on bodyweight and a favourable long-term bodyweight profile. Preliminary studies indicate that there is a high level of patient acceptability and satisfaction with quetiapine.
In conclusion, quetiapine has shown efficacy against both positive and negative symptoms of schizophrenia, and has benefits in improving cognitive deficits, affective symptoms and aggression/hostility. The beneficial effects of quetiapine have been maintained for at least 52 weeks. Quetiapine was effective and well tolerated in hard-to-treat patients, and may be of particular use in these individuals. It is at least as effective as standard antipsychotics and appears to have similar efficacy to risperidone and olanzapine. The relative risk/benefit profile of quetiapine compared with other atypical antipsychotics requires further research in head-to-head trials, although quetiapine’s relatively benign tolerability profile distinguishes it from other commonly used atypical agents, particularly with respect to bodyweight, EPS and plasma prolactin levels. Overall, quetiapine has an excellent risk/benefit profile and is a suitable first-line option for the treatment of schizophrenia.
Overview of Pharmacodynamic Properties
Quetiapine is a dibenzothiazepine derivative, with a relatively higher binding affinity for serotonin 5-HT2 receptors (drug concentration required for 50% inhibition of radioligand binding [IC50] 148 nmol/L) than for dopamine D2 receptors (IC50 329 nmol/L). The drug’s negligible affinity for muscarinic receptors suggests a low risk for anticholinergic adverse effects.
In animal models, quetiapine has shown clozapine-like antipsychotic activity predictive of efficacy against both positive and negative symptoms, a low liability to induce extrapyramidal symptoms (EPS) and a reduced propensity to affect prolactin levels. Furthermore, in vitro studies of limbic selectivity suggest that the pharmacological effects of quetiapine are selective for the mesolimbic and mesocortical dopamine systems (responsible for antipsychotic effects), but not the nigrostriatal dopamine system (responsible for EPS).
Overview of Pharmacokinetic Properties
Oral quetiapine (75–250mg every 8 hours) is rapidly absorbed and demonstrates dose-proportional increases in maximum steady-state plasma concentrations and areas under the plasma concentration-time curves from 0 to 8 hours at steady state. Absorption is not significantly affected by food. Quetiapine has a large apparent volume of distribution and is approximately 83% plasma protein bound.
Quetiapine is extensively metabolised in the liver; the major metabolic pathway is sulphoxidation by cytochrome P450 (CYP) 3A4. The drug is excreted primarily in the urine (≈73%) as inactive metabolites. The elimination half-life of quetiapine is approximately 6 hours.
The pharmacokinetics of quetiapine do not differ in men compared with women, or in adolescents compared with adults and appear unaffected by cigarette smoking or ethnic background. In contrast, apparent oral clearance was up to 50% lower in elderly (aged 63–85 years) than in younger patients (aged 18–43 years). Patients with hepatic cirrhosis or severe renal impairment (creatinine clearance 8–33 mL/min/1.73m2) had reduced (by ≈25%) mean oral clearance compared with healthv controls.
Quetiapine may potentially interact with drugs that are potent inducers (e.g. phenytoin, carbamazepine, barbiturates, rifampicin or glucocorticoids) or inhibitors (e.g. ketoconazole, itraconazole, fluconazole and erythromycin) of CYP3A4.
Therapeutic Efficacy
In short-term, randomised, double-blind trials in patients with acute schizophrenia, quetiapine (up to 750 mg/day) was generally significantly more effective than placebo at improving psychopathology (Brief Psychiatric Rating Scale [BPRS] total score, p ≤ 0.05; Clinical Global Impression [CGI] scales, p ≤ 0.01). The drug has shown efficacy against both positive (improvement in BPRS positive symptoms cluster score, p = 0.003 for quetiapine ≤750 mg/day) and negative (measured using Scale for the Assessment of Negative Symptoms, p ≤ 0.05) symptoms of schizophrenia versus placebo. Responses observed with quetiapine were clinically meaningful (defined as an improvement of ≥30% from baseline in BPRS total score) in approximately 50% of recipients.
Overall, quetiapine (up to 750 mg/day) was at least as effective as chlorpromazine (mean 384 mg/day) and had similar efficacy to haloperidol (up to 16 mg/day) in the management of patients with acute schizophrenia in randomised, double-blind trials of 6–12 weeks’ duration (measured using BPRS and Positive and Negative Symptom Scale [PANSS] total scores, and response rates). The similar efficacy of quetiapine to haloperidol in this patient group was confirmed in a meta-analysis of four double-blind trials. Furthermore, quetiapine 600 mg/day was at least as effective as haloperidol 20 mg/day in the treatment of patients with schizophrenia nonresponsive or partially responsive to previous antipsychotic treatment (fluphenazine titrated to 20 mg/day for 4 weeks); indeed, clinical response (improvement of ≥20% in PANSS score) was higher in quetiapine than in haloperidol recipients (52.2% vs 38.0%, p = 0.043). In addition, quetiapine (up to 750 mg/day) was effective in the management of schizophrenia in patients who were switched from standard (e.g. haloperidol) or atypical antipsychotic agents (including olanzapine and risperidone) following an inadequate response or intolerance in a noncomparative trial; most patients switched to quetiapine had a decrease of ≥1 point on the Index of Clinical Benefit, and significant improvements from baseline were observed at week 12 in PANSS and CGI Severity of Illness scores (both p < 0.001).
Interim results suggest similar efficacy for quetiapine (600 mg/day), olanzapine (15 mg/day) and risperidone (5 mg/day) in a small, randomised, rater-blinded trial. Furthermore, improvements in overall psychopathology and the positive and negative symptoms of schizophrenia were similar with quetiapine (up to 800 mg/ day) to those with risperidone (up to 8 mg/day) in a double-blind trial in patients with acute schizophrenia, as were response rates (26.5% and 26.9%) in this trial, or in a nonblind trial in patients with psychoses. In this latter trial, there were no significant differences between treatments in a subgroup of patients with schizophrenia.
In a preliminary analysis, quetiapine improved symptoms and overall psychopathology in a small number of patients with first-episode schizophrenia.
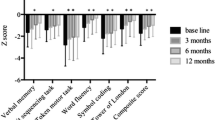
Improvements with quetiapine were maintained for up to 130 weeks in adult patients with schizophrenia and for at least 52 weeks in elderly patients with the disease in open-label, follow-up studies. In randomised, double-blind trials in patients with schizophrenia, quetiapine also had benefits in improving cognitive function (especially verbal reasoning and fluency) compared with haloperidol (p < 0.05) and depressive symptoms compared with placebo or risperidone. Posthoc analyses suggest that quetiapine (150–750 mg/day) is effective compared with placebo in the treatment of patients displaying hostility and aggression (p < 0.001 for reduction in Behavioural Agitation Scores).
Tolerability
Quetiapine is well tolerated in patients with schizophrenia. Dizziness, orthostatic hypotension, dry mouth and dyspepsia were the only adverse events with an incidence of ≥5% that occurred in at least twice as many quetiapine as placebo recipients in a pooled analysis of 3- to 6-week placebo-controlled trials. Most adverse events were mild to moderate in intensity. There was no significant difference between quetiapine and placebo groups in overall incidence of treatment discontinuation due to adverse events. The tolerability of quetiapine in long-term trials was similar to that in short-term trials.
Quetiapine has a low propensity to induce EPS in patients with schizophrenia in both short- and long-term treatment. Importantly, placebo-level EPS occurred across all quetiapine dosages tested (75–750 mg/day) in a fixed-dose study. EPS occurred with a significantly lower incidence in quetiapine than in haloperidol or chlorpromazine recipients with schizophrenia, and quetiapine had a more favourable EPS profile than risperidone in patients with DSM-IV psychoses; quetiapine recipients were less likely to experience substantial EPS or receive concurrent medication for EPS than risperidone recipients (both p < 0.001). Current data suggest that quetiapine has a low risk for EPS in vulnerable patient groups, including the elderly, adolescents and patients with organic brain disorders; however, additional research is needed to confirm these predominantly preliminary results.
Unlike risperidone and amisulpride, quetiapine (across its therapeutic dose range) does not appear to be associated with sustained increases in plasma prolactin levels; indeed, reductions in prolactin levels were noted in many patients in the quetiapine clinical trials programme. Furthermore, quetiapine may normalise prolactin levels in patients previously treated with antipsychotics. The placebo-level effect of quetiapine on plasma prolactin levels is reflected in the low incidence of hormonal and sexual adverse events in the quetiapine clinical trials programme.
Quetiapine appears to have a minimal short-term effect on bodyweight and a favourable effect on bodyweight in the long term. There was little change in corrected QT intervals and no clinically significant changes in clinical chemistry parameters with quetiapine therapy in clinical trials. In addition, agranulocytosis or lens changes have not been causally linked to quetiapine treatment.
Long-term treatment with quetiapine was associated with good patient acceptability and satisfaction in an open-label multicentre study using a nonvalidated questionnaire.
Pharmacoeconomic Considerations
Limited pharmacoeconomic analyses suggest that, despite its relatively high acquisition cost, quetiapine may not add to the overall costs of treating schizophrenia and has the potential to be cost saving, although additional research is required. Results from a Markov model (constructed, in part, from a randomised, double-blind trial in patients nonresponsive or partially responsive to previous antipsychotic therapy) indicate that, over 5 years, the higher acquisition cost of quetiapine relative to haloperidol was offset by lower costs for other medications, inpatient hospitalisations and outpatient care; thus, the two treatments had similar overall per-patient costs (£38 106 vs £38 350; year of costing not reported). Indirect costs were not considered in this analysis, which was from the perspective of the UK National Health Service.
Quetiapine was the dominant treatment relative to risperidone, achieving greater effectiveness for less cost in a cost-utility analysis of clinical data from a 4-month, randomised, open-label study in outpatients with schizophrenia or other psychotic disorders. Average daily costs for quetiapine and risperidone were SUS6.38 and $US7.85; incremental gains in utilities (0.23 vs 0.12; last-observation-carried-forward analyses) equated to an average incremental gain in quality-adjusted life years for quetiapine recipients of 3.85 years per patient (p < 0.05).
Dosage and Administration
In adult patients with schizophrenia, quetiapine is licensed to dosages of 750 or 800 mg/day. The target dosage of quetiapine recommended in the manufacturer’s prescribing information is 300–400 or 450 mg/day, although the manufacturer more recently suggests that the initial target dosage should be 600 mg/day to achieve optimum response. A faster rate of titration (increments of 200 mg/day) than that recommended in the current prescribing information (25–50mg twice daily) may be appropriate in acutely ill patients. Patients responding to quetiapine should receive the lowest effective dosage for maintenance therapy.
A slower rate of titration and lower target dose should be considered in elderly patients and those with a predisposition to hypotensive reactions. An initial dosage of 25 mg/day is recommended in patients with hepatic impairment, with subsequent increments of 25–50 mg/day until an effective dosage is reached.








Similar content being viewed by others
Notes
The use of tradenames is for product identification purposes only and does not imply endorsement.
References
American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: American Psychiatric Association, 1994
American Psychiatric Association Working Group on Schizophrenia. Practice guideline for the treatment of patients with schizophrenia. Am J Psychiatry 1997 Apr; 154: 1–63
Koreen AJ, Siris SG, Chakos M, et al. Depression in firstepisode schizophrenia. Am J Psychiatry 1993 Nov; 150(11): 1643–8
Keck JPE, Strakowski SM, McElroy SL. The efficacy of atypical antipsychotics in the treatment of depressive symptoms, hostility, and suicidality in patients with schizophrenia. J Clin Psychiatry 2000; 61Suppl. 3: 4–9
Bridler R, Umbricht D. Atypical antipsychotics in the treatment of schizophrenia. Swiss Med Wkly 2003 Feb 8; 133(5–6): 63–76
Kasper S, Müller-Spahn F. Review of quetiapine and its clinical applications in schizophrenia. Expert Opin Pharmacother 2000 May; 1(4): 783–801
Gunasekara NS, Spencer CM. Quetiapine: a review of its use in schizophrenia. CNS Drugs 1998 Apr; 9(4): 325–40
Fulton B, Goa KL. ICI-204,636: an initial appraisal of its pharmacological properties and clinical potential in the treatment of schizophrenia. CNS Drugs 1995; 4(1): 68–78
Goldstein JM. Preclinical profile of Seroquel (quetiapine): an atypical antipsychotic with clozapine-like pharmacology. In: Holliday SG, Ancill RJ, MacEwan GW, editors. Schizophrenia: breaking down the barriers. Chichester: John Wiley & Sons Ltd, 1996: 177–236
Casey DE. Seroquel (quetiapine): preclinical and clinical findings of a new atypical antipsychotic. Expert Opin Invest Drugs 1996; 5(8): 939–57
Nemeroff CB, Kinkead B, Goldstein J. Quetiapine: preclinical studies, pharmacokinetics, drug interactions, and dosing. J Clin Psychiatry 2002; 63Suppl. 13: 5–11
Kasper S, Tauscher J, Willeit M, et al. Receptor and transporter imaging studies in schizophrenia, depression, bulimia and Tourette’s disorder: implications for psychopharmacology. World J Biol Psychiatry 2002 Jul; 3(3): 133–46
Gefvert O, Bergstrom M, Langstrom B, et al. Time course of central nervous dopamine-D2 and 5-HT2 receptor blockade and plasma drug concentrations after discontinuation of quetiapine (Seroquel) in patients with schizophrenia. Psychopharmacology (Berl) 1998 Jan; 135(2): 119–26
Kapur S, Zipursky R, Jones C, et al. A positron emission tomography study of quetiapine in schizophrenia: a preliminary finding of an antipsychotic effect with only transiently high dopamine D2 receptor occupancy. Arch Gen Psychiatry 2000; 57(6): 553–9
Goldstein J. Mechanism of action of quetiapine: a modulator of dopamine at the D2 receptor [abstract]. Schizophr Res 2003; 60(1 Suppl. 5): 311
Ellenbroek BA, Lubbers LJ, Cools AR. Activity of ‘Seroquel’ (ICI 204,636) in animal models for atypical properties of antipsychotics: a comparison with clozapine. Neuropsycho-pharmacology 1996 Oct; 15(10): 406–16
Migler BM, Warawa EJ, Malick JB. Seroquel: behavioral effects in conventional and novel tests for atypical antipsychotic drug. Psychopharmacology (Berl) 1993; 112(2–3): 299–307
Guan HJ, Dai J, Zhu XZ. Atypical antipsychotic effects of quetiapine fumarate in animal models. Acta Pharmacol Sin 2000 Mar; 21(3): 205–10
Goldstein JM, Litwin LC, Sutton EB, et al. Seroquel: electrophysiological profile of a potential atypical antipsychotic. Psychopharmacology (Berl) 1993; 112: 293–8
Robertson GS, Matsumura H, Fibiger HC. Induction patterns of Fos-like immunoreactivity in the forebrain as predictors of atypical antipsychotic activity. J Pharmacol Exp Ther 1994 Nov; 271(2): 1058–66
Vahid-Ansari F, Nakabeppu Y, Robertson GS. Contrasting effects of chronic clozapine, Seroquel (ICI 204,636) and haloperidol administration on delta fosB-like immunoreactivity in the rodent forebrain. Eur J Neurosci 1996 May; 8(5): 927–36
Goldstein J, Arvanitis L. ICI204, 636 (Seroquel): a dibenzothiazepine atypical antipsychotic. Review of preclinical pharmacology and highlights of phase II clinical trials. CNS Drug Rev 1995; 1: 50–73
Sailer CF, Salama AI. Seroquel: biochemical profile of a potential atypical antipsychotic. Psychopharmacology (Berl) 1993; 112(2–3): 285–92
DeVane CL, Nemeroff CB. Clinical pharmacokinetics of quetiapine: an atypical antipsychotic. Clin Pharmacokinet 2001; 40(7): 509–22
Grimm SW, Stams KR, Bui K. In vitro prediction of potential metabolic drug interactions for Seroquel [abstract]. Schizophr Res 1997; 24(1/2): 198
Thyrum PT, Fabre LF, Wong YWJ. Multiple-dose pharmacokinetics of ICI 204,636 in schizophrenic men and women [abstract]. Psychopharmacol Bull 1996; 32(3): 525
Thyrum PT, Jaskiw G, Fuller M, et al. Multiple-dose pharmacokinetics of ICI 204,636 in elderly patients with selected psychotic disorders [abstract]. Psychopharmacol Bull 1996; 32(3): 524
Wong YW, Ewing BJ, Thyrum PT, et al. Multiple-dose pharmacokinetics of Seroquel (quetiapine) in schizophrenia men and women [abstract]. Schizophr Res 1997; 24: 200
AstraZeneca. Seroquel® (quetiapine fumate) tablets. Wilmington (DE): 2001
McConville BJ, Arvanitis LA, Thyrum PT, et al. Pharmacokinetics, tolerability and clinical effectiveness of quetiapine fumarate: an open-label trial in adolescents with psychotic disorders. J Clin Psychiatry 2000 Apr; 61(4): 252–60
Thyrum PT, Wong YWJ, Yeh C. Single-dose pharmacokinetics of quetiapine in subjects with renal or hepatic impairment. Prog Neuropsychopharmacol Biol Psychiatry 2000 May; 24(4): 521–33
AstraZeneca. Combined summary of product characteristics: Seroquel [online]. Available from URL: http://www.astrazeneca.co.uk [Accessed 2003 Nov 5]
Wong YWJ, Yeh C, Thyrum PT. The effects of concomitant phenytoin administration on the steady-state pharmacokinetics of quetiapine. J Clin Psychopharmacol 2001; 21(1): 89–93
Potkin SG, Thyrum PT, Bera R, et al. Open-label study of the effect of combination quetiapine/lithium therapy on lithium pharmacokinetics and tolerability. Clin Ther 2002 Nov; 24(11): 1809–23
Potkin SG, Thyrum PT, Alva G, et al. The safety and pharmacokinetics of quetiapine when coadministered with haloperidol, risperidone, or thioridazine. J Clin Psychopharmacol 2002 Apr; 22(2): 121–30
Potkin SG, Thyrum PT, Alva G, et al. Effect of fluoxetine and imipramine on the pharmacokinetics and tolerability of the antipsychotic quetiapine. J Clin Psychopharmacol 2002 Apr; 22(2): 174–82
Arvanitis LA, Miller BG, Seroquel Trial 13 Study Group. Multiple fixed doses of Seroquel (quetiapine) in patients with acute exacerbation of schizophrenia: a comparison with haloperidol and placebo. Biol Psychiatry 1997 Aug 15; 42(4): 233–46
Borison RI, Arvanitis LA, Miller BG. ICI 204,636, an atypical antipsychotic: efficacy and safety in a multicenter, placebo-controlled trial in patients with schizophrenia. US Seroquel Study Group. J Clin Psychopharmacol 1996 Apr; 16(2): 158–69
Small JG, Hirsch SR, Arvanitis LA, et al. Quetiapine in patients with schizophrenia: a high- and low-dose double-blind comparison with placebo. Arch Gen Psychiatry 1997 Jun; 54(6): 549–57
Murasaki M, Koyama T, Yagi G, et al. Efficacy and tolerability of quetiapine compared with haloperidol in patients with schizophrenia [abstract no. P.01.215]. Int J Neuropsychopharmacol 2000 Jul; 3 Suppl. 1: 150
Copolov DL, Link CGG, Kowalcyk B. A multicentre, double-blind, randomized comparison of quetiapine (ICI 204,636, ‘Seroquel’) and haloperidol in schizophrenia. Psychol Med 2000 Jan; 30(1): 95–105
Peuskens J, Link CGG. A comparison of quetiapine and chlor-promazine in the treatment of schizophrenia. Acta Psychiatr Scand 1997 Oct; 96(4): 265–73
Mullen J, Jibson MD, Sweitzer D, et al. A comparison of the relative safety, efficacy, and tolerability of quetiapine and risperidone in outpatients with schizophrenia and other psychotic disorders: the Quetiapine Experience with Safety and Tolerability (QUEST) Study. Clin Ther 2001 Nov; 23(11): 1839–54
Zhong X, Sweitzer D, Potter L, et al. A comparison of the efficacy and safety of quetiapine and risperidone [abstract no. NR530]. American Psychiatric Association 2003 Annual Meeting: New Research Abstracts; 2003 May 17–22; San Francisco: 198
Emsley RA, Raniwalla J, Bailey PJ, et al. A comparison of the effects of quetiapine (‘Seroquel’) and haloperidol in schizophrenic patients with a history of and a demonstrated, partial response to conventional antipsychotic treatment: Prize Study Group. Int Clin Psychopharmacol 2000 May; 15(3): 121–31
Rak IW, Westhead EK, Raniwalla J, et al. Maintenance of long-term efficacy with ‘Seroquel®’ (quetiapine) [poster]. Annual Meeting of the American College of Neuropsycopharmacology; 1999 Dec 12–16; Acapulco
Kasper S. Maintenance of long-term efficacy and safety of quetiapine in schizophrenia [abstract]. American Psychiatric Association Annual Meeting; 2001 May 5; New Orleans
Vinogradov S, Salzman C, Pultz JA, et al. Long-term safety, tolerability and clinical improvement with quetiapine in elderly patients with schizophrenia [abstract no. B.86]. Schizophr Res 2000; 41(1): 207
Tariot PN, Salzman C, Yeung PP, et al. Long-term use of quetiapine in elderly patients with psychotic disorder. Clin Ther 2000 Sep; 22(9): 1068–84
De Nayer A, Windhager E, Irmansyah X, et al. Efficacy and tolerability of quetiapine in patients with schizophrenia switched from other antipsychotics. Int J Psychiatry Clin Pract 2003; 7: 59–70
Larmo I. Quetiapine improves symptoms and is well tolerated in patients with schizophrenia switched from olanzapine [abstract no. P.2.078]. Plus poster presented at the 16th Congress of the European College of Neuropsychopharmacology; 2003 Jun 19-22; Vienna. Eur Neuropsychopharmacol 2003; 13Suppl. 4: S313
De Nayer A, Jones AM, Whiteford JL, et al. Switching to quetiapine improves CGI and PANSS scores in patients with schizophrenia [abstract no. P.2.W.020]. Plus poster presented at the 16th Congress of the European College of Neuropsychopharmacology; 2003 Sep 20–24; Prague. Int J Neuropsychopharmacol 2002; 5Suppl. 1: 122
Windhager E, Sayce R. Switching to quetiapine in patients with schizophrenia inadequately responsive to or intolerant of risperidone [abstract plus poster]. World Psychiatric Association Meeting; 2003 Jun 19–22; Vienna
Shaw JA, Lewis JE, Pascal S, et al. A study of quetiapine: efficacy and tolerability in psychotic adolescents. J Child Adolesc Psychopharmacol 2001 Winter; 11(4): 415–24
McConville B, Carrero L, Sweitzer D, et al. Long-term safety, tolerability, and clinical efficacy of quetiapine in adolescents: an open-label extension trial. J Child Adolesc Psychopharmacol 2003 Spring; 13(1): 75–82
Anon. EFESEN: Seroquel (quetiapine) effective and well-tolerated in first-episode schizophrenia [online]. Available from URL: http://www.docguide.com/news/content [Accessed 2003 Dec 8]
Good KP, Kiss I, Buiteman C, et al. Improvement in cognitive functioning in patients with first-episode psychosis during treatment with quetiapine: an interim analysis. Br J Psychiatry 2002 Sep; 181Suppl. 43: S45–9
Fleming K, Kalali A, Yeh C, et al. The neurocognitive effects of quetiapine (Seroquel, ICI 204636) [abstract]. Schizophrenia Res 1997; 24: 197
Sax KW, Strakowski SM, Keck PE. Attentional improvement following quetiapine fumarate treatment in schizophrenia. Schizophr Res 1998 Oct 9; 33(3): 151–5
Velligan DI, Prihoda TJ, Sui D, et al. The effectiveness of quetiapine versus conventional antipsychotics in improving cognitive and functional outcomes in standard treatment settings. J Clin Psychiatry 2003 May; 64(5): 524–31
Purdon SE, Malla A, Labelle A, et al. Neuropsychological change in patients with schizophrenia after treatment with quetiapine or haloperidol. J Psychiatry Neurosci 2001 Mar; 26(2): 137–49
Velligan DI, Newcomer J, Pultz J, et al. Does cognitive function improve with quetiapine in comparison to haloperidol? Schizophr Res 2002 Jan 15; 53(3): 239–48
Hellewell J, Cantillion M, McKellar M, et al. ‘Seroquel’: efficacy in aggression, hostility and low mood of schizophrenia [abstract]. Int J Neuropsychopharmacol 1999; 2Suppl. 1: S112
Emsley R, Oosthuizen P. The new and evolving pharmacotherapy of schizophrenia. Psychiatr Clin North Am 2003 Mar; 26(1): 141–63
Chengappa KN, Goldstein JM, Greenwood M, et al. A post hoc analysis of the impact on hostility and agitation of quetiapine and haloperidol among patients with schizophrenia. Clin Ther 2003 Feb; 25(2): 530–41
Horacek J. The effect of quetiapine on cognitive functions and negative symptoms [abstract]. 55th Institute on Psychiatric Services for the American Psychiatric Association; 2003 Oct 29–Nov 2; Boston
Schulz SC, Thomson R, Brecher M. The efficacy of quetiapine vs. haloperidol and placebo: a meta-analytic study of efficacy. Schizophr Res 2003 Jul 1; 62(1–2): 1–12
Zhong X, Sweitzer D, Russo J, et al. To compare the efficacy, safety, and tolerability of quetiapine and risperidone in treating patients with schizophrenia [abstract no. P.2.140]. Eur Neuropsychopharmacol 2003; 13Suppl. 4: S340
Sacchetti E, Valsecchi P, Regini C, et al. Comparison of quetiapine, olanzapine and risperidone in patients with schizophrenia: interim results of a randomised, rater-blinded study [abstract no. P.2.162]. Plus poster presented at the 16th Congress of the European College of Neuropsycopharmacology; 2003 Sep 20–24; Prague. Eur Neuropsychopharmacol 2003; 13Suppl 4: S350
Tandon R, Mullen J, Sweitzer D. Quetiapine and risperidone in outpatients with schizophrenia: subanalysis of the QUEST trial [poster no. NR242]. American Psychiatric Association Annual Meeting; 2001 May 5–10; New Orleans
Tandon R, Jibson MD. Efficacy of newer generation antipsychotics in the treatment of schizophrenia. Psychoneuroendo-crinology 2003 Jan; 28Suppl. 1: 9–26
Tafalla M, Herranz S. Long-term treatment with quetiapine is effective and well tolerated in outpatients with schizophrenia [abstract]. Deutsche Gesellschaft für Psychiatrie, Psychotherapie und Nervenheilkunde; 2003 Nov 19–23; Berlin
Kasper S. Maintenance of long-term efficacy and safety of quetiapine in the treatment of schizophrenia [poster no. NR239]. American Psychiatric Association Annual Meeting; 2001 May 5–10; New Orleans
Csernansky JG, Schuchart EK. Relapse and rehospitalisation rates in patients with schizophrenia: effects of second generation antipsychotics. CNS Drugs 2002; 16(7): 473–84
Taylor DM, Duncan-McConnell D. Refractory schizophrenia and atypical antipsychotics. J Psychopharmacol 2000; 14(4): 409–18
Emsley RA. Role of newer atypical antipsychotics in the management of treatment resistant schizophrenia. CNS Drugs 2000 Jun; 13(6): 409–20
Pilowsky LS, Ohlsen RI, O’Toole M, et al. Quetiapine treatment of first episode psychosis: the Southwark first onset psychosis service (FIRST). A preliminary audit of psychotic symptoms. European First Episode Schizophrenia Network Meeting; 2002 Feb 23; Davos
Kasper S, Resinger E. Cognitive effects and antipsychotic treatment. Psychoneuroendocrinology 2003 Jan; 28Suppl. 1: 27–38
Sajatovic M, Mullen JA, Sweitzer DE. Efficacy of quetiapine and risperidone against depressive symptoms in outpatients with psychosis. J Clin Psychiatry 2002 Dec; 63(12): 1156–63
Emsley R, Bucklay P, Jones AM, et al. Differential effect of quetiapine on depressive symptoms in patients with partially responsive schizophrenia. J Psychopharmacol 2003 Jun; 17(2): 210–5
Goldstein JM. Quetiapine fumarate (Seroquel®): a new atypical antipsychotic. Drugs Today 1999 Mar; 35(3): 193–210
Hellewell JS. Quetiapine: a well-tolerated and effective atypical antipsychotic. Hosp Med 2002 Oct; 63(10): 600–3
Hamner MB. Acute and long-term effects of quetiapine on plasma prolactin levels [abstract no. 418]. Biol Psychiatry 2001 Apr 15; 49(8 Suppl.): 120–1
Hamner MB, McConville BJ. Quetiapine does not produce sustained elevations of prolactin levels [abstract no. NR508]. American Psychiatric Association Annual Meeting; 2001 May 5; New Orleans: 259
Jones AM, Rak IW, Raniwalla J, et al. Weight changes in patients treated with quetiapine [abstract no. NR712]. American Psychiatric Association 2000 Annual Meeting; 2000 May 13–18; Chicago
Arvanitis LA, Rak IW. The long-term efficacy and safety of ‘Seroquel’ (quetiapine) [abstract]. Schizophrenia Res 1997; 24: 196–7
Juncos J, Yeung P, Sweitzer D, et al. Quetiapine improves psychotic symptoms associated with Parkinson’s disease [abstract no. NR656]. American Psychiatric Association Annual Meeting; 1999 May 17–22; Washington, DC
Mintzer JE, Yeung P, Mullen JA, et al. EPS variations in the elderly [abstract no. NR663]. American Psychiatric Association 2000 Annual Meeting; 2000 May 13–18; Chicago: 13
Parsa MA, Greenway HM, Bastini B. Treatment of psychosis in patients with Parkinson’s disease and dementia (Lewy body disease variant) with quetiapine [abstract]. World Psychiatric Association Annual Meeting; 1999 Aug 6–11; Hamburg
Goldstein JM, Arvanitis LA, Cantillon M. Low incidence of reproductive/hormonal side effects with ‘Seroquel’ (quetiapine fumarate) is supported by its lack of elevation of plasma prolactin concentrations. 36th Annual Meeting of the American College of Neuropsychopharmacology; 1997 Dec 8–12; Waikoloa
Platz T, Jones AM, Whiteford JL, et al. Drug tolerability after switching to quetiapine in schizophrenia [abstract no. P.2.W021]. Int J Neuropsychopharmacol 2002 Jun; 5Suppl. 1: 123
Larmo I, Jones AM, Whiteford JL, et al. Switching to quetiapine reduces EPS in patients with schizophrenia [abstract no. P.3.W.024]. Int J Neuropsychopharmacol 2002 Jun; 5Suppl. 1: 164
Westhead EK, Jones AM, Gorman AP. Long-term effect of quetiapine on weight in patients with schizophrenia receiving no concomitant antipsychotic medication [poster]. 22nd Collegium Internationale Neuropsychpharmacologicum Congress; 2000 Jul 9–13; Brussels
Glazer WM, Morgenstern H, Pultz JA, et al. Incidence of persistent tardive dyskinesia may be lower with quetiapine treatment than previously reported with typical antipsychotics in patients with psychoses [poster]. Annual Meeting of the American College of Neuropsycopharmacology; 1999 Dec 12–16; Acapulco
Nagy J. Effectiveness of quetiapine up to 1600 mg/day: short-term results with 14-month follow-up [abstract no. P. 2.139]. Plus poster presented at the 16th Congress of the European College of Neuropsychopharmacology; 2003 Sep 20–24; Prague. Eur Neuropsychopharmacol; 2003 13Suppl. 4: S340
Hellewell JSE, Westhead E. Safety during long-term exposure to quetiapine [abstract no. NR238]. American Psychiatric Association Annual Meeting; 2001 May 5–10; New Orleans: 441
Trenton A, Currier G, Zwemer F. Fatalities associated with therapeutic use and overdose of atypical antipsychotics. CNS Drugs 2003; 17(5): 307–24
Smith MA, McCoy R, Hamer J, et al. NR568: optimal titration for quetiapine: a pilot study [poster]. Annual Meeting of the American Psychiatric Association; 2003 May 17–22; San Francisco
Taylor DM. Antipsychotics and QT prolongation. Acta Psychiatr Scand 2003 Feb; 107(2): 85–95
Atkinson GA, Hellewell JSE, Raniwalla J. Extrapyramidal symptom and tolerability profile of Seroquel® (quetiapine): an overview of phase II/III placebo controlled trials [abstract no. P.2.100]. Eur Neuropsychopharmacol 1997 Sep; 7Suppl. 2: 224
Meats P. Quetiapine (‘Seroquel’): an effective and well-tolerated atypical antipsychotic. Int J Psychiatry Clin Pract 1997; 1: 231–9
Gianfrancesco F, White R, Wang R-H, et al. Antipsychotic-induced type 2 diabetes: evidence from a large health plan database. J Clin Psychopharmacol 2003; 23(4): 328–34
Citrome LL, Jaffe AB. Relationship of atypical antipsychotics with development of diabetes mellitus. Ann Phamacother 2003; 37: 1849–57
Laties AM. Quetiapine and cataracts [letter]. Am J Psychiatry 2002 Feb; 159(2): 322–3
Laties AM, Dev V, Geller W, et al. Safety update on lenticular opacities: benign experience with 620,000 US patient exposures to quetiapine [poster]. 2001 International Congress on Schizophrenia Research; 2001 Apr 28–May 2; Whistler
Jann MW, Cohen JL. Economic considerations and formulary management of oral antipsychotics. Dis Manage Health Outcomes 1998 Mar; 3(3): 115–29
Weiden PJ, Ashworth P. Switching from fluphenazine to quetiapine ameliorates extrapyramidal symptoms and neuroendocrine side effects in patients with schizophrenia [abstract no. P.2.046]. Eur Neuropsychopharmacol 2001 Oct; 11Suppl. 3: 262–3
Jeste DV, Glazer WM, Morgenstern H, et al. Rarity of persistent tardive dyskinesia with quetiapine treatment of psychotic disorders in the elderly [poster]. Annual Meeting of the American College of Neuropsychopharmacology; 1999 Dec 12–16; Acapulco
Meaney AM, O’Keane V. Prolactin and schizophrenia: clinical consequences of hyperprolactinaemia. Life Sci 2002 Jul 19; 71(9): 979–92
Halbreich U, Kinon BJ, Gilmore JA, et al. Elevated prolactin levels in patients with schizophrenia: mechanisms and related adverse effects. Psychoneuroendocrinology 2003 Jan; 28Suppl. 1: 53–67
Bobes J, Garc A-Portilla MP, Rejas J, et al. Frequency of sexual dysfunction and other reproductive side-effects in patients with schizophrenia treated with risperidone, olanzapine, quetiapine, or haloperidol: the results of the EIRE study. J Sex Marital Ther 2003 Mar–Apr 30; 29(2): 125–47
Knegtering R, Castelein S, Bous H, et al. A randomized open-label study of the impact of quetiapine versus risperidone on sexual functioning [abstract no. P.2.301]. 16th Congress of the European College of Neuropsychopharmacology; 2003 Sep 20–24; Prague
Nasrallah H. A review of the effect of atypical antipsychotics on weight. Psychoneuroendocrinology 2003 Jan; 28Suppl. 1: 83–96
Altaian CA, Brecher M, Rak IW, et al. The effects of long-term quetiapine monotherapy on weight in patients with schizophrenia [abstract no. 137]. Biol Psychiatry 2001 Apr 15; 49(8 Suppl.): 53
Brecher M, Rak IW, Melvin K, et al. The long-term effect of quetiapine (Seroquel™) monotherapy on weight in patients with schizophrenia. Int J Psychiatry Clin Pract 2000; 4(4): 287–91
Bobes J, Rejas J, Garcia-Garcia M, et al. Weight gain in patients with schizophrenia treated with risperidone, olanzapine, quetiapine or haloperidol: results of the EIRE study. Schizophr Res 2003 Jul 1; 62(1–2): 77–88
Kalali A. Quality of life: the patients subjective experience of psychotrophics: results of an international study [abstract no. STH0405]. Int J Neuropsychopharmacol 1998 Jul; 1Suppl. 1: 81–2
Cupillari M. Use of quetiapine in psychotic disorders: evaluation of the effects of the treatment using the drug attitude inventory 30 (DAI30) [abstract no. P.2.021]. Plus poster presented at the 16th Congress of the European College of Neuro-psychopharmacology; 2003 Sep 20–24; Prague. Eur Neuropsychopharmacol 2003; 13Suppl. 4: S287
Hellewell JSE, Kalali AH, Langham SJ, et al. Patient satisfaction and acceptability of long-term treatment with quetiapine. Int J Psychiatry Clin Pract 1999; 3(2): 105–13
Hellewell JSE. Patients’ subjective experiences of antipsychotics: clinical relevance. CNS Drugs 2002; 16(7): 457–71
Foster RH, Goa KL. Olanzapine: a pharmacoeconomic review of its use in schizophrenia. Pharmacoeconomics 1999 Jun; 15(6): 611–40
Sartorius N, Fleischhacker WW, Gjerris A, et al. The usefulness and use of second-generation antipsychotic medications. Curr Opin Psychiatry 2002; 15Suppl. 1: S1–51
Tilden D, Aristides M, Meddis D, et al. An economic assessment of quetiapine and haloperidol in patients with schizophrenia only partially responsive to conventional antipsychotics. Clin Ther 2002 Oct; 24(10): 1648–67
Simons WR, Meddis D. Cost-utility analysis of quetiapine compared to risperidone in the treatment of patients with schizophrenia or other psychotic disorders [abstract no. PMH27]. Value Health 2001; 4(2): 149
Simons WR, Meddis D. A health economic evaluation of quetiapine compared with risperidone: a supplemental analysis of the QUEST trial [poster]. The International Society for Pharmacoeconomics and Outcomes Research (ISPOR) 6th Annual International Meeting; 2001 May 20–23; Arlington
AstraZeneca. Seroquel prescribing information [online]. Available from URL: http://www.psychiatry-in-practice.com [Accessed 2003 Dec 3]
Keks NA, Tonso MA, Tabone K, et al. Experience with atypical antipsychotics in an acute inpatient unit [abstract]. Schizophr Res 2003; 60(1 Suppl. 1): 289
Sicuro F. Use of higher-dose quetiapine in elderly inpatients: a chart review study [abstract]. Schizophr Res 2003; 60 (1 Suppl. 1): 315
AstraZeneca. Macclesfield: AstraZeneca, 2003. (Data on file)
Treatment of schizophrenia 1999: the expert consensus guidelines series. J Clin Psychiatry 1999; 60 Suppl. 11: 3–80
National Institute for Clinical Excellence. Guidance on the use of newer (atypical) antipsychotic drugs for the treatment of schizophrenia. London: National Institute for Clinical Excellence. Technology Appraisal Guidance 2002 Jun; Report no.: 43
Dixon L, Lehman A, Levine J. Conventional antipsychotic medications for schizophrenia. Schizophr Bull 1995; 21(4): 567–78
Goren JL, Levin GM. Quetiapine, an atypical antipsychotic. Pharmacotherapy 1998 Nov–Dec 31; 18(6): 1183–94
Sajatovic M, Madhusoodanan S, Buckley P. Schizophrenia in the elderly: guidelines for management. CNS Drugs 2000 Feb; 13(12): 103–15
Adams C, Wilson P, Gilbody S, et al. Drug treatments for schizophrenia. Qual Health Care 2000 Mar; 9(1): 73–9
Wagstaff A, Bryson H. Clozapine: a review of its pharmacological properties and therapeutic use in patients with schizophrenia who are unresponsive to or intolerant of classical antipsychotic agents. CNS Drugs 1995; 4(5): 370–400
Kelleher JP, Centorrino F, Albert MJ, et al. Advances in atypical antipsychotics for the treatment of schizophrenia: new formulations and new agents. CNS Drugs 2002; 16(4): 249–61
Hamner M. The effects of atypical antipsychotics on serum prolactin levels. Ann Clin Psychiatry 2002 Sep; 14(3): 163–73
Brown CS, Markowitz JS, Moore TR, et al. Atypical antipsychotics. Part II: adverse effects, drug interactions, and costs. Ann Pharmacother 1999 Feb; 33(2): 210–7
Author information
Authors and Affiliations
Corresponding author
Additional information
Various sections of the manuscript reviewed by: J. Bobes, Depamento de Psiquiatria, Universidad de Oviedo, Spain; R. Emsley, Department of Psychiatry, University of Stellenbosch, Cape Town, South Africa; J.S.E. Hellewell, Trafford General Hospital, Manchester, UK; S. Kasper, Department of General Psychiatry, University of Vienna, Vienna, Austria; D.A. Revicki, MEDTAP International Inc, Bethesda, Maryland, USA; D.M. Taylor, Pharmacy Department, Maudsley Hospital, London, UK; D.I. Velligan, Department of Psychiatry, University of Texas Health Science Center, San Antonio, Texas, USA; J. Peuskens, Universitair Centrum, Kortenberg, Belgium.
Data Selection
Sources: Medical literature published in any language since 1980 on quetiapine, identified using Medline and EMBASE, supplemented by AdisBase (a proprietary database of Adis International). Additional references were identified from the reference lists of published articles. Bibliographical information, including contributory unpublished data, was also requested from the company developing the drug. Search strategy: Medline and EMBASE search terms were ‘quetiapine’ and ‘schizophrenia’. AdisBase search terms were (‘quetiapine’ or ‘ICI 204636’) and ‘schizophrenia’. Searches were last updated 22 January 2003. Selection: Studies in patients with schizophrenia who received quetiapine. Inclusion of studies was based mainly on the methods section of the trials. When available, large, well controlled trials with appropriate statistical methodology were preferred. Relevant pharmacodynamic and pharmacokinetic data are also included.
Index terms: Quetiapine, schizophrenia, pharmacodynamics, pharmacokinetics, therapeutic use.
Rights and permissions
About this article
Cite this article
Cheer, S.M., Wagstaff, A.J. Quetiapine. CNS Drugs 18, 173–199 (2004). https://doi.org/10.2165/00023210-200418030-00004
Published:
Issue Date:
DOI: https://doi.org/10.2165/00023210-200418030-00004