Abstract
Background: Recently developed German guidelines for antiviral treatment in patients with chronic hepatitis C recommend basing drug dosage, intended treatment duration and early stopping rules on the genotype of the hepatitis C virus and early viral responses to treatment.
Objectives: To evaluate effectiveness and cost effectiveness of different antiviral treatment strategies including the German guidelines, for chronic hepatitis C.
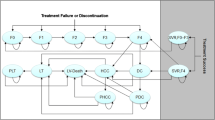
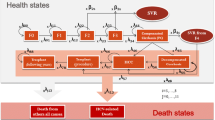
Methods: A validated lifetime Markov model was used to project life expectancy, QALYs and lifetime costs for the following strategies: (i) no antiviral therapy (NoAVT); (ii) interferon-α-2b plus ribavirin for 48 weeks (IFN + R); (iii) peginterferon-α-2b plus weight-based ribavirin for 48 weeks (PEG + R); (iv) peginterferon-α-2b plus ribavirin according to German guidelines with genotype-dependent treatment duration, dosage and 12-week viral response evaluation (GUIDE). Clinical and resource utilization data were derived from a clinical trial, the published literature and a survey of German hepatologists. Incremental cost-effectiveness ratios (ICERs) were calculated adopting the German societal perspective. Costs (in €, year 2005 values) and health outcomes were discounted at 3% annually. Uncertainty was assessed using deterministic and probabilistic sensitivity analyses.
Results: Compared with NoAVT, PEG+R increased undiscounted life expectancy by 5.0 life-years (5.2 QALYs) and GUIDE increased undiscounted life expectancy by 4.9 years (5.1 QALYs). Compared with PEG + R, GUIDE saved 13% of hepatitis C virus-related lifetime costs per patient. GUIDE dominated IFN + R. Compared with NoAVT, discounted ICERs were €1500 per QALY for GUIDE and €3200 per QALY for PEG + R.
Conclusion: Administering GUIDE should allow tailoring treatment efficiently to genotype, bodyweight and early viral response in patients with chronic hepatitis C, and appears cost effective compared with other well accepted medical interventions.







Similar content being viewed by others
Reference
Bennett WG, Inoue Y, Beck JR, et al. Estimates of the cost-effectiveness of a single course of interferon-alpha 2b in patients with histologically mild chronic hepatitis C. Ann Intern Med 1997; 127 (10): 855–65
Wong JB, Poynard T, Ling MH, et al. Cost-effectiveness of 24 or 48 weeks of interferon alpha-2b alone or with ribavirin as initial treatment of chronic hepatitis C. International Hepatitis Interventional Therapy Group. Am J Gastroenterol 2000; 95 (6): 1524–30
Younossi ZM, Singer ME, McHutchison JG, et al. Cost effectiveness of interferon alpha2b combined with ribavirin for the treatment of chronic hepatitis C. Hepatology 1999; 30 (5): 1318–24
Shepherd J, Waugh N, Hewitson P. Combination therapy (interferon alfa and ribavirin) in the treatment of chronic hepatitis C: a rapid and systematic review. Health Technol Assess 2000; 4 (33): 1–67
Stein K, Rosenberg W, Wong J. Cost effectiveness of combination therapy for hepatitis C: a decision analytic model. Gut 2002; 50 (2): 253–8
Buti M, Casado MA, Fosbrook L, et al. Cost-effectiveness of combination therapy for naive patients with chronic hepatitis C. J Hepatol 2000; 33 (4): 651–8
Sennfalt K, Reichard O, Hultkrantz R, et al. Cost-effectiveness of interferon alfa-2b with and without ribavirin as therapy for chronic hepatitis C in Sweden. Scand J Gastroenterol 2001; 36 (8): 870–6
Sagmeister M, Wong J, Mullhaupt B, et al. A pragmatic and cost-effective strategy of a combination therapy of interferon alpha-2b and ribavirin for the treatment of chronic hepatitis C. Eur J Gastroenterol Hepatol 2001; 13 (5): 483–8
Siebert U, Sroczynski G, Rossol S, et al. Cost-effectiveness of peginterferon alpha-2b plus ribavirin versus interferon alpha-2b plus ribavirin for initial treatment of chronic hepatitis C. Gut 2003; 52: 425–32
Sullivan SD, Craxi A, Alberti A, et al. Cost effectiveness of peginterferon alpha-2a plus ribavirin versus interferon alpha-2b plus ribavirin as initial therapy for treatment-naive chronic hepatitis C. Pharmacoeconomics 2004; 22 (4): 257–65
Buti M, Medina M, Casado MA, et al. A cost-effectiveness analysis of peginterferon alfa-2b plus ribavirin for the treatment of naive patients with chronic hepatitis C. Aliment Pharmacol Ther 2003; 17 (5): 687–94
Siebert U, Sroczynski G, with the collaboration of the German Hepatitis C Model (GEHMO) Group. Antiviral therapy in patients with chronic hepatitis C in Germany: clinical and economic evaluation of the initial combination therapy with interferon/peginterferon and ribavirin. Health Technology Assessment. Vol. 8. HTA series of the German Institute for Medical Documentation and Information, commissioned by the Federal Ministry of Health and Social Security. Cologne: German Institute for Medical Documentation and Information (DIMDI), 2003
Siebert U, Sroczynski G. Effectiveness and cost-effectiveness of initial combination therapy with interferon/peginterferon plus ribavirin in patients with chronic hepatitis C in Germany: a health technology assessment commissioned by the German Federal Ministry of Health and Social Security. Int J Tech Assess Health Care 2004; 21 (1): 55–65
Shepherd J, Brodin H, Cave C, et al. Pegylated interferon alpha-2a and -2b in combination with ribavirin in the treatment of chronic hepatitis C: a systematic review and economic evaluation. Health Technol Assess 2004; 8 (39): iii-iv, 1–125
Shepherd J, Brodin HF, Cave CB, et al. Clinical- and cost-effectiveness of pegylated interferon alfa in the treatment of chronic hepatitis C: a systematic review and economic evaluation. Int J Technol Assess Health Care 2005; 21 (1): 47–54
National Institutes of Health. National Institutes of Health Consensus Development Conference Statement: management of hepatitis C. 2002: June 10–12, 2002. Hepatology 2002; 36 (5 Suppl. 1): S3–20
Seeff LB, Hoofnagle J. National Institutes of Health Consensus Development Conference: management of hepatitis C. 2002. Hepatology 2002; 36 (5 Suppl. 1): S1–2
Zeuzem S. Standard treatment of acute and chronic hepatitis C. Z Gastroenterol 2004; 42 (8): 714–9
Beck JR, Pauker SG. The Markov process in medical prognosis. Med Decis Making 1983; 3: 419–58
Sonnenberg FA, Beck JR. Markov models in medical decision making: a practical guide. Med Decis Making 1993; 13: 322–38
Knodell RG, Ishak KG, Black WC, et al. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology 1981; 1 (5): 431–5
Desmet VJ, Gerber M, Hoofnagle JH, et al. Classification of chronic hepatitis: diagnosis, grading, and staging. Hepatology 1994; 19: 1513–20
Yousuf M, Nakano Y, Sodeyama T, et al. Persistence of viremia in patients with type-C chronic hepatitis during long-term follow-up. Scand J Gastroenterol 1992; 27: 812–6
Tremolada F, Casarin C, Alberti A, et al. Long-term follow-up of non-A, non-B (type C) post-transfusion hepatitis. J Hepatol 1992; 16: 273–81
Mattsson L. Outcome of acute symptomatic non-A, non-B hepatitis: a 13-year follow-up study of hepatitis C virus markers. Liver 1993; 13: 274–8
Takahashi M, Yamada G, Miyamoto R, et al. Natural course of chronic hepatitis C. Am J Gastroenterol 1993; 88 (2): 240–3
Fattovich G, Giustina G, Degos F, et al. Morbidity and mortality in compensated cirrhosis type C: a retrospective follow-up study of 384 patients. Gastroenterology 1997; 112 (2): 463–72
Salerno F, Borroni G, Moser P, et al. Survival and prognostic factors of cirrhotic patients with ascites: a study of 134 outpatients. Am J Gastroenterol 1993; 88 (4): 514–9
Anonymous. Sclerotherapy for male alcoholic cirrhotic patients who have bled from esophageal varices: results of a randomized, multicenter clinical trial. The Veterans Affairs Cooperative Variceal Sclerotherapy Group. Hepatology 1994; 20 (3): 618–25
Christensen E, Krintel JJ, Hansen SM, et al. Prognosis after the first episode of gastrointestinal bleeding or coma in cirrhosis: survival and prognostic factors. Scand J Gastroenterol 1989; 24 (8): 999–1006
Ascher N, Lake J, Emond J, et al. Liver transplantation for hepatitis C virus-related cirrhosis. Hepatology 1994; 20 (1 Pt 2): 24S-7S
Detre K, Belle S, Lombarddero M. Liver transplantation for chronic viral hepatitis. Viral Hepatitis Rev 1996; 2: 219–28
Kilpe V, Krakauer H, Wren R. An analysis of liver transplant experience from 37 transplant centers as reported to Medicare. Transplantation 1993; 56 (3): 554–61
Fischer-Fröhlich CL. Organ transplantation in the Federal Republic of Germany and in Europe from a medical perspective: an evaluation of the situation [in German]. In: Landeszentrale für politische Bildung Baden-Württemberg, editor. Organentnahme und Transplantation-im Spannungsfeld zwischen Ethik und Gesetz. Bad Urach/Stuttgart, 1997: 7–28
Berg J, Bechstein WO, Mueller A, et al. Lebertransplantation. Internist (Berl) 1998; 39: 1237–45
Deutsche Stiftung Organtransplantation (DSO). Figures and data: donated and transplanted organs 1992–2000 [online]. Available from URL: http://www.dso.de/ [Accessed 2002 Jan 30]
Federal Statistical Office Germany. Life tables for Germany 2003–2005 [online]. Available from URL: http://www.destatis.de/2006 [Accessed 2007 Feb 27]
Manns M, McHutchison J, Gordon S, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet 2001; 358 (9286): 958–65
Drummond MF, O’Brien B, Stoddart GL, et al. Methods for the economic evaluation of health care programmes. 2nd ed. New York: Oxford University Press, 1997
Gold MR, Siegel JE, Russell LB, et al. Cost-effectiveness in health and medicine. New York: Oxford University Press, 1996
Siebert U, Ravens-Sieberer U, Greiner W, et al. Patient-based health-related quality of life in different health stages of chronic hepatitis C [abstract]. Hepatology 2001; 44 (2): 222A
Siebert U, Ravens-Sieberer U, Greiner W, et al. Performance of different utility assessment methods in chronic hepatitis C patients. In: Kind P, Macran S, editors. Proceedings of the 19th Plenary Meeting of the EuroQol Group 13th-14th September 2002 Discussion Papers. York: Centre for Health Economics, University of York, 2003: 175–84
Heckerling PS, Verp MS. Amniocentesis or chorionic villus sampling for prenatal genetic testing: a decision analysis. J Clin Epidemiol 1991; 44 (7): 657–70
Kuppermann M, Shiboski S, Feeny D, et al. Can preference scores for discrete states be used to derive preference scores for an entire path of events? An application to prenatal diagnosis. Med Decis Making 1997; 17 (1): 42–55
German Red List [database on the Internet]. PegIntron, p. 10, 2006 [online]. Available from URL: http://www.rote-liste.de [Accessed 2009 Mar 4]
Anonymous. Gebührenordnung für Ärzte (GOÄ) [online]. Available from URL: http://www.e-bis.de/goae/defaultFrame.htm 2002 [Accessed 2002 Jul 27]
Kassenärztliche Bundesvereinigung. Einheitlicher Bewertungsmaßstab (EBM) [online]. Available from URL: http://www.kbv.de/8156.html, 2002 [Accessed 2002 Jul 27]
Federal Statistical Office Germany. Price index for physicians, hospitals and other health care services. Wiesbaden: Statistisches Bundesamt, 2003
Maddrey WC. Safety of combination interferon alfa-2b/ribavirin therapy in chronic hepatitis C-relapsed and treatment-naive patients. Semin Liver Dis 1999; 19 Suppl. 1: 67–75
Welte R, Leidl R. Übertragung der Ergebnisse ökonomischer Evaluationsstudien aus dem Ausland auf Deutschland: Probleme und Lösungsansätze. Ansätze und Methoden der ökonomischen Evaluation – eine internationale Perspektive. In: Leidl R, Schulenburg JM, Wasem J, editors. Baden-Baden: Nomos, 1999; 81–112
Drummond MF, Bloom BS, Carrin G, et al. Issues in the cross-national assessment of health technology. Int J Technol Assess Health Care 1992; 8: 671–82
Baltussen B, Ament A, Leidl R. Making cost assessments based on RCTs more useful to decision-makers. Health Policy 1996; 37: 163–83
Anonymous. Lauer-Taxe Arzneimittelverzeichnis: Apothekeneinkaufs- und verkaufspreise [online]. Available from URL: http://www.lauer-fischer.de/LF/Seiten/Produkte/Online-Dienste/Lauer-Taxe%20online.aspx 2002 [Accessed 2002 Mar 12]
Luce BR, Manning WG, Siegel JE, et al. Estimating costs in cost-effectiveness analysis. In: Gold MR, Siegel JE, Russell LB, et al., editors. Cost-effectiveness in health and medicine. New York: Oxford University Press, 1996: 176–213
Weinstein MC, Siegel JE, Gold MR, et al. Recommendations of the Panel on Cost-effectiveness in Health and Medicine. JAMA 1996; 276 (15): 1253–8
Dore G, Freeman A, Law M, et al. Is severe liver disease a common outcome for people with chronic hepatitis C? J Gastroenterol Hepatol 2002; 17 (4): 423–30
Freeman A, Dore G, Law M, et al. Estimating progression to cirrhosis in chronic hepatitis C virus infection. Hepatology 2001; 34 (4): 809–16
Bonis PA, Ioannidis JP, Cappelleri JC, et al. Correlation of biochemical response to interferon alfa with histological improvement in hepatitis C: a meta-analysis of diagnostic test characteristics. Hepatology 1997; 26 (4): 1035–44
Reichard O, Glaumann H, Fryden A, et al. Two-year biochemical, virological, and histological follow-up in patients with chronic hepatitis C responding in a sustained fashion to interferon alfa-2b treatment. Hepatology 1995; 21 (4): 918–22
Marcellin P, Asselah T, Boyer N. Fibrosis and disease progression in hepatitis C. Hepatology 2002; 36 (5 Suppl.): S47–56
Weinstein MC. High-priced technology can be good value for money. Ann Intern Med 1999; 130 (10): 857–8
Wong JB, Sonnenberg FA, Salem DN, et al. Myocardial revascularization for chronic stable angina: analysis of the role of percutaneous transluminal coronary angioplasty based on data available in 1989. Ann Intern Med 1990; 113 (11): 852–71
Wong JB, Davis GL, McHutchison JG, et al. Economic and clinical effects of evaluating rapid viral response to peginterferon alfa-2b plus ribavirin for the initial treatment of chronic hepatitis C. Am J Gastroenterol 2003; 98 (11): 2354–62
Siebert U. When should decision-analytic modeling be used in the economic evaluation of health care? Eur J Health Econ 2003; 4 (3): 143–50
Siebert U. Using decision-analytic modelling to transfer international evidence from health technology assessment to the context of the German Health Care System. A HTA Methods Report. (Series of the German Institute for Medical Documentation and Information commissioned by the Federal Ministry of Health and Social Security). Köln: DIMDI, 2005
Acknowledgements
Further members of the following research groups participated in this study:
Participating members of the German Hepatitis C Model (GEHMO) Group: F. Buchner (Munich Re); M. Bullinger (University of Hamburg, Berlin); Annette Conrads-Frank (Harvard Medical School, Boston, MA); W. Greiner (University of Hanover); F. Hessel (University of Duisburg-Essen); Bärbel M. Kurth (Robert Koch-Institute, Berlin); Georg Marckmann (University of Tübingen); J.M. Graf von der Schulenburg (University of Hanover); Ulrike Ravens-Sieberer (Robert Koch-Institute, Berlin), Silke Siebert (University of Munich, Germany).
Participating members of the International Hepatitis Interventional Therapy (IHIT) Group: F.H. Anderson (Vancouver General Hospital, Vancouver, BC, Canada); S. Arora (University of New Mexico, Albuquerque, NM, USA); B. Bacon (St. Louis University School of Medicine, St. Louis, MO, USA); L. Balart (Center for Digestive Diseases, New Orleans, LA, USA); K.G. Benner (Oregon Health Sciences University, Portland, OR, USA); M.-A. Bigard (Hopital de Brabois Adultes, Vandoeuvre Les Nancy, France); H.C. Bodenheimer (Mt. Sinai Medical Center, New York, NY, USA); M. Bourliere (Hopital Saint Joseph, Marseille, France); C. Brechot (Hôpital Necker, Paris, France); H. Brunner (KH Lainz der Stadt Wien, Vienna, Austria); S. Caldwell (University of Virginia, Charlottesville, VA, USA); W. Carey (Cleveland Clinic Foundation, Cleveland, OH, USA); R.L. Carithers Jr (University of Washington, Seattle, WA, USA); G.L. Davis (University of Florida, Gainesville, FL, USA); J. Dienstag (Massachusetts General Hospital, Boston, MA, USA); J. Donovan (University of Nebraska, Omaha, NE, USA); R. Esteban-Mur (Hospital Valle d’Hebron, Barcelona, Spain), M. Buti (Hospital Valle d’Hebron, Barcelona, Spain); G.T. Everson (University of Colorado, Denver, CO, USA); S. Feinman (Mount Sinai Hospital, Toronto, ON, Canada); S. Flamm (Northwestern Memorial Hospital, Chicago, IL, USA); P.R. Galle (Klinikum der Johannes-Gutenberg-Universität, Mainz, Germany); R. Gish (California Pacific Medical Center, San Francisco, CA, USA); N. Gitlin (Emory University, Atlanta, GA, USA); T. Goeser (Medizinische Einrichtungen der Universität Köln, Germany); S. Gordon (William Beaumont Hospital, Royal Oak, MI, USA); H. Greten (Universitäts-Krankenhaus Eppendorf, Hamburg, Germany); S. Hadzyiannis (Hippokration Hospital, Athens, Greece); I. Hokeberg (Akademiska Hospital, Uppsala, Sweden); I. Jacobson (Cornell University, New York, NY, USA); P. Kwo (Indiana University School of Medicine, Indianapolis, IN, USA); D.R. LaBrecque (University of Iowa Hospital and Clinic, Iowa City, IA, USA); W.M. Lee (University of Texas Southwestern Medical Center, Dallas, TX, USA); S. Lindgren (University Hopital MAS, Malmö, Sweden); K.L. Lindsay (University of Southern California, Los Angeles, CA, USA); M.P. Manns (Medizinische Hochschule Hannover, Hannover, Germany); P. Marcellin (Hôpital Beaujon, Clichy, France); P. Marotta (London Health Sciences Centre, University, London, ON, Canada); T. McGarrity (Pennsylvania State University, Hershey, PA, USA); J.G. McHutchison (Scripps Clinic, La Jolla, CA, USA); R. Moreno (Hospital de la Princesa, Madrid, Spain); T.R. Morgan (VA Medical Center, Long Beach, CA, USA); R. Perrillo (Ochsner Clinic, New Orleans, LA, USA); M. Poliquin (Centre Universitaire de l’Universite de Mtl, Montreal, QC, Canada); Thierry Poynard (Hôpital Pitié-Salpiêtrière, Paris, France); J. Rakela (University of Pittsburgh, Pittsburgh, PA, USA); R. Reindollar (Charlotte Clinic for GI and Liver Diseases, Charlotte, NC, USA); J.L. Rodriguez-Agullo (Hospital Clinico Universitario San Carlos, Madrid, Spain); R. Rouzier-Panis (Centre CAP, Nime, France); V. Rustgi (Metropolitan Research, Fairfax, VA, USA); J.M. Sanchez-Tapias (Hospital Clinic I Provincial, Barcelona, Spain); E.R. Schiff (University of Miami School of Medicine, Miami, FL, USA); D. Schuppan (Klinikum der Universität Erlangen-Nürnberg, Erlangen, Germany); M. Sherman (The Toronto Hospital, Toronto, ON, Canada); M.L. Shiffman (Medical College of Virginia, Richmond, VA, USA); M. Silva (Fundacion Favaloro, Buenos Aires, Argentina); C. Smith (Minnesota Clinical Research Center, St. Paul, MN, USA); H. Tanno (Clinica del Higado, Rosario, Argentina); C. Trepo (Hospital Hotel Dieu, Lyon, France); W. Vogel (Leopold-Franzens-University Innsbruck, Innsbruck, Austria); T. Wright (University of California San Francisco, San Francisco, CA, USA); S. Zeuzem (Klinikum der J.W. Goethe Universität, Frankfurt, Germany).
Panel of expert German hepatologists participating in the survey of German practice: C.H. Antoni (University Hospital, Mannheim); T.H. Berg (University Hospital Charite, Berlin); N. Demmel (Teaching Hospital of the University of Munich, Städtisches Krankenhaus München-Neuperlach); D. Hüppe (Herne); B. Kallinowski (University of Heidelberg); C. Kölbel (Teaching Hospital of the University of Mainz, Trier); S. Mauss (Düsseldorf); B. Moeller (Berlin); M.K. Müller (Marienhospital, Osnabrück); C. Niederau (Academic Teaching Hospital of the University of Essen, St. Josefs Hospital, Oberhausen); G. Teuber (University Hospital, Frankfurt); L. Theilmann (Städtisches Klinikum, Pforzheim); E. Will (Mannheim); R. Zachoval (Großhadern Medical Center, University of Munich); A. Zipf (Mannheim).
The authors also acknowledge the advice of members of the Advisory Board of the German Hepatitis C Model (GEHMO) Group: R. Holle (Institute of Health Economics and Health Care Management, GSF-National Research Centre for Environment and Health, Neuherberg, Germany); N. Mühlberger (University of Munich, Germany); B. Gibis (Institute of Health Economics, Edmonton, AL, Canada).
The authors received an unrestricted research grant from Schering Plough Corporation, Kenilworth, NJ, USA and funding for a national Health Technology Assessment commissioned by the Agency of Health Technology Assessment at the German Institute for Medical Documentation and Information (DAHTA@DIMDI) [No. 05/01.2.] This study was sponsored by an unrestricted research grant from Schering Plough Corporation, Kenilworth, NJ, USA. The authors had complete and independent control over study design, analysis and interpretation of data, report writing and publication, regardless of results.
U. Siebert and G. Sroczynski have received funding from different health technology assessment agencies to perform health technology assessments related to hepatitis C. (i.e., DAHTA@DIMDI — German Agency for Health Technology Assessment at the German Institute Medical Documentation and Information, German Federal Ministry of Health; CADTH — Canadian Agency for Drugs and Technologies in Health; ITA — Institute for Technology Assessment at the Austrian Academy of Sciences, LBIHTA, Ludwig Boltzmann Institute for Health Technology Assessment, Austria). They have both also received unrestricted research grants from Schering Plough and Roche to perform studies related to hepatitis. C.J. Wasem has received grants/honoraria from a number of pharmaceutical companies, including Schering Plough, Novartis and Valeant. He did not receive any specific funding for this paper. J. McHutchison has acted as a consultant and scientific advisor for Schering Plough, and has received grant support from Schering Plough. M. Manns has received grants from and acted as a consultant to a number of pharmaceutical companies including Schering Plough, Novartis, Roche and Valeant.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Siebert, U., Sroczynski, G., Aidelsburger, P. et al. Clinical Effectiveness and Cost Effectiveness of Tailoring Chronic Hepatitis C Treatment with Peginterferon Alpha-2b Plus Ribavirin to HCV Genotype and Early Viral Response. Pharmacoeconomics 27, 341–354 (2009). https://doi.org/10.2165/00019053-200927040-00006
Published:
Issue Date:
DOI: https://doi.org/10.2165/00019053-200927040-00006