Abstract
Introduction: Gastrointestinal (GI) complications are common following renal transplantation. Discontinuing or reducing the dosage of mycophenolate mofetil can improve GI tolerability but adversely affect graft outcomes. This analysis was undertaken to assess the 3-year economic and clinical impact of mycophenolate mofetil dosage modifications or discontinuation following post-transplant GI events compared with no dosage modification.
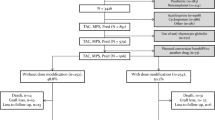
Methods: Adult renal transplant recipients with a Medicare-covered mycophenolate mofetil prescription at the time of GI complication between 1995 and 2000 were drawn from the US Renal Data System (USRDS). The 3-year graft survival rates after first diagnosis of a GI complication were obtained in four cohorts of patients according to mycophenolate mofetil administration within 6 months of initial GI diagnosis: (i) no dosage change in mycophenolate mofetil (NC); (ii) one or more episodes of mycophenolate mofetil dosage reduction ≥50% of the initial dosage, lasting >30 days (DR ≥50%); (iii) one or more episodes of mycophenolate mofetil dosage reduction <50% of the initial dosage, lasting >30 days (DR ≥50%); and (iv) one or more episodes of mycophenolate mofetil discontinuation >30 days (DC).
Two multivariate models were used to estimate the association between DR and DC and graft survival <6 months after GI diagnosis and 6–36 months after diagnosis. In each cohort, Medicare costs for maintaining a patient with stable function were calculated using regression and were augmented with cost of graft failure, resumed maintenance dialysis and death post-graft loss using Medicare data supplied by the USRDS. Survival and cost outcomes were integrated in a 3-year Markov model with 6-month cycles. The perspective was that of Medicare, and costs and outcomes were discounted by 3% per annum.
Results: Adult patients (n = 3589) with a mycophenolate mofetil prescription at time of diagnosis of GI event were identified: NC = 2230 (62.1%); DR <50% = 247 (6.9%); DR ≥50% = 348 (9.7%); and DC = 764 (21.3%). In the first 6 months after GI diagnosis, DC was associated with increased risk of graft failure (hazard ratio [HR] 3.20; 95% CI 1.71, 5.99; p < 0.0001). During the period 6–36 months after GI diagnosis, the HR for graft loss was higher for the DR ≥50% group (HR 1.32; 95% CI 1.02, 1.70; p < 0.05) and DC group (HR 1.35; 95% CI 1.09, 1.69; p < 0.01) relative to the NC group.
Expected 3-year cumulative Medicare costs per patient were $US68 495 for the NC and DR <50% groups, $US70 886 for the DR ≥50% group, $US79 015 for the DC group and $US70 967 overall. Respective QALYs were 2.32, 2.30, 2.27 and 2.31. In sensitivity analysis, reducing the rate of DR and DC by 25% would have lowered expected costs by $US2.2 million in the study population and increased QALYs by 11.2. Monte Carlo simulation indicated a 93% probability that such reduction in the relative risk of mycophenolate mofetil DR/DC was cost saving or cost neutral.
Conclusion: Dosage reduction or discontinuation of mycophenolate mofetil in the first 6 months after diagnosis of GI complications is associated with significantly increased risk of graft failure and increased healthcare costs in adult renal transplant recipients.








Similar content being viewed by others
References
Kalo Z, Jaray J, Nagy J. Economic evaluation of kidney transplantation versus hemodialysis in patients with end-stage renal disease in Hungary. Prog Transplant 2001 Sep; 11 (3): 188–93
Laupacis A, Keown P, Pus N, et al. A study of the quality of life and cost-utility of renal transplantation. Kidney Int 1996; 50: 235–42
Mutinga N, Brennan D, Schnitzler MA. Consequences of eliminating HLA-B in deceased donor kidney allocation to increase minority transplantation. Am J Transplant 2005; 5: 1090–8
Wolfe RA, Ashby VB, Milford EL, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med 1999 Dec 2; 341 (23): 1725–30
Halloran PF. Immunosppressive drugs for kidney transplantation. N Engl J Med 2004; 351: 2715–29
Keown P. Improving quality of life: the new target for transplantation. Transplantation 2001 Dec 27; 72 (12 Suppl.): S67–74
Meier-Kriesche HU, Schold JD, Srinivas TR, et al. Lack of improvement in renal allograft survival despite a marked decrease in acute rejection rates over the most recent era. Am J Transplant 2004 Mar; 4 (3): 378–83
Santiago-Delpin EA, Morales-Otero LA, Gonzalez ZA. Gastrointestinal complications and appendicitis after kidney transplantation. Transplant Proc 1989 Aug; 21 (4): 3745–8
Kahan BD. Efficacy of sirolimus compared with azathioprine for reduction of acute renal allograft rejection: a randomised multicentre study. The Rapamune US Study Group. Lancet 2000 Jul 15; 356 (9225): 194–202
Pirsch JD, Miller J, Deierhoi MH, et al. A comparison of tacrolimus (FK506) and cyclosporine for immunosuppression after cadaveric renal transplantation. FK506 Kidney Transplant Study Group. Transplantation 1997 Apr 15; 63 (7): 977–83
Rossi SJ, Schroeder TJ, Hariharan S, et al. Prevention and management of the adverse effects associated with immunosuppressive therapy. Drug Saf 1993 Aug; 9 (2): 104–31
Troppmann C, Papalois BE, Chiou A, et al. Incidence, complications, treatment, and outcome of ulcers of the upper gastrointestinal tract after renal transplantation during the cyclosporine era. J Am Coll Surg 1995 Apr; 180 (4): 433–43
Meyers WC, Harris N, Stein S, et al. Alimentary tract complications after renal transplantation. Ann Surg 1979 Oct; 190 (4): 535–42
European Mycophenolate Mofetil Cooperative Study Group. Placebo-controlled study of mycophenolate mofetil combined with cyclosporin and corticosteroids for prevention of acute rejection. European Mycophenolate Mofetil Cooperative Study Group. Lancet 1995 May 27; 345 (8961): 1321–5
Sollinger HW. Mycophenolate mofetil for the prevention of acute rejection in primary cadaveric renal allograft recipients. US Renal Transplant Mycophenolate Mofetil Study Group. Transplantation 1995 Aug 15; 60 (3): 225–32
Allison AC, Eugui EM. Mycophenolate mofetil and its mechanisms of action. Immunopharmacology 2000 May; 47 (2–3): 85–118
Ojo AO, Meier-Kriesche HU, Hanson JA, et al. Mycophenolate mofetil reduces late renal allograft loss independent of acute rejection. Transplantation 2000 Jun 15; 69 (11): 2405–9
Bunnapradist S, Lentine KL, Burroughs TE, et al. Mycophenolate mofetil dose reductions and discontinuations after gastrointestinal complications are associated with renal transplant graft failure. Transplantation 2006 Jul 15; 82 (1): 102–7
Hardinger KL, Brennan DC, Lowell J, et al. Long-term outcome of gastrointestinal complications in renal transplant patients treated with mycophenolate mofetil. Transpl Int 2004 Nov; 17 (10): 609–16
Knoll GA, MacDonald I, Khan A, et al. Mycophenolate mofetil dose reduction and the risk of acute rejection after renal transplantation. J Am Soc Nephrol 2003 Sep; 14 (9): 2381–6
Pelletier RP, Akin B, Henry ML, et al. The impact of mycophenolate mofetil dosing patterns on clinical outcome after renal transplantation. Clin Transplant 2003 Jun; 17 (3): 200–5
Tierce JC, Porterfield-Baxa J, Petrilla AA, et al. Impact of mycophenolate mofetil (mycophenolate mofetil)-related gastrointestinal complications and mycophenolate mofetil dose alterations on transplant outcomes and healthcare costs in renal transplant recipients. Clin Transplant 2005 Dec; 19 (6): 779–84
United States Renal Data System. Researcher’s guide to the USRDS database. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases, 2004
Beck JR, Pauker SG. The Markov process in medical prognosis. Med Decis Making 1983; 3 (4): 419–58
Briggs A, Sculper M. An introduction to Markov modelling for economic evaluation. Pharmacoeconomics 1998; 13 (4): 397–409
Whiting JF. Standards for economic and quality of life studies in transplantation. Transplantation 2000 Oct 15; 70 (7): 1115–21
Yen EF, Hardinger K, Brennan DC, et al. Cost-effectiveness of extending Medicare coverage of immunosuppressive medications to the life of a kidney transplant. Am J Transplant 2004 Oct; 4 (10): 1703–8
Whiting JF, Kiberd B, Kalo Z, et al. Cost-effectiveness of organ donation: evaluating investment into donor action and other donor initiatives. Am J Transplant 2004 Apr; 4 (4): 569–73
Hornberger JC, Best JH, Garrison Jr LP. Cost-effectiveness of repeat medical procedures: kidney transplantation as an example. Med Decis Making 1997; 17: 363–72
Gold MR, Siegel JE, Russell LB, et al., editors. Cost-effectiveness in health and medicine. 1st ed. New York: Oxford University Press, 1996
Consumer price index. Washington, DC: US Bureau of Labor Statistics, 2002
Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53: 457–81
Pasta DJ, Cisternas MG. Estimating standard errors for CLASS variables in generalized linear models using PROC IML [paper 264–28]. Sugi 26[online]. Available from URL: http://www2.sas.com/proceedings/sugi28/264-28.pdf [Accessed 2007 Nov 15]
Callahan M, Battleman DS, Christos P, et al. Economic consequences of renal dysfunction among cardiopulmonary bypass surgery patients: a hospital-based perspective. Value Health 2003 Mar-Apr; 6 (2): 137–43
Zhan C, Miller MR. Excess length of stay, charges, and mortality attributable to medical injuries during hospitalization. JAMA 2003 Oct 8; 290 (14): 1868–74
Kleinman L, Faull R, Walker R, et al. Gastrointestinal-specific patient reported outcome instruments differentiate between renal transplant patients with or without GI complications. Transplant Proc 2005; 37 (2): 846–9
Briggs AH. Handling uncertainty in cost-effectiveness models. Pharmacoeconomics 2000 May; 17 (5): 479–500
Fenwick E, O’Brien BJ, Briggs A. Cost-effectiveness acceptability curves: facts, fallacies and frequently asked questions. Health Econ 2004 May; 13 (5): 405–15
Hariharan S, Johnson CP, Bresnahan BA, et al. Improved graft survival after renal transplantation in the United States, 1988 to 1996. N Engl J Med 2000 Mar 2; 342 (9): 605–12
The Tricontinental Mycophenolate Mofetil Renal Transplantation Group. A blinded, randomized clinical trial of mycophenolate mofetil for the prevention of acute rejection in cadaveric renal transplantation. Transplantation 1996 Apr 15; 61 (7): 1029–37
Behrend M. Adverse gastrointestinal effects of mycophenolate mofetil: aetiology, incidence and management. Drug Saf 2001; 24 (9): 645–63
Stirnemann PM, Takemoto SK, Schnitzler MA, et al. Agreement of immunosuppression regimens described in Medicare pharmacy claims with the Organ Procurement and Transplantation Network survey. J Am Soc Nephrol 2006 Aug; 17 (8): 2299–306
Meier-Kriesche HU, Li S, Gruessner RW, et al. Immunosuppression: evolution in practice and trends, 1994–2004. Am J Transplant 2006; 6 (5 Pt 2): 1111–31
Lin DY. Linear regression analysis of censored medical costs. Biostatistics 2000 Mar; 1 (1): 35–47
Chan L, Mulgaonkar S, Walker R, et al. Patient-reported gastrointestinal symptom burden and health-related quality of life following conversion from mycophenolate mofetil to enteric-coated mycophenolate sodium. Transplantation 2006 May 15; 81 (9): 1290–7
Acknowledgements
Data reported here were supplied by the United States Renal Data System (USRDS). The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the US Government.
Supported in part by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases (K25-DK-02916-03, Mark A. Schnitzler, PhD, P.I.). Additional funding was provided by Novartis Pharma AG, Basel, Switzerland.
Gerardo Machnicki is an employee of Novartis Pharmaceuticals Corporation, USA, and Jean-Francois Ricci is an employee of Novartis Pharma AG, Switzerland. Novartis is the manufacturer of Myfortic®, a competitor drug of mycophenolate mofetil, the drug discussed in this paper. Daniel Brennan and Mark Schnitzler have received funding for research from a variety of companies including Novartis, Roche and Astellas.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Machnicki, G., Ricci, JF., Brennan, D.C. et al. Economic Impact and Long-Term Graft Outcomes of Mycophenolate Mofetil Dosage Modifications Following Gastrointestinal Complications in Renal Transplant Recipients. Pharmacoeconomics 26, 951–967 (2008). https://doi.org/10.2165/00019053-200826110-00007
Published:
Issue Date:
DOI: https://doi.org/10.2165/00019053-200826110-00007