Abstract
Background
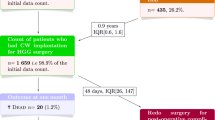
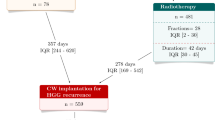
High-grade gliomas are aggressive brain tumours that are extremely challenging to treat effectively. The intracranial implantation of carmustine wafers (BCNU-W), which delivers chemotherapy directly to the affected area, may prolong survival in this population. However, no attention has yet been paid to the economic implications of BCNU-W in this setting.
Objective
To investigate the cost effectiveness of BCNU-W as an adjunct to surgery followed by radiotherapy, compared with surgery plus radiotherapy alone. Newly diagnosed, operable grade III and IV gliomas in a population with a mean age of 55 years were considered.
Methods
A Markov cost-utility model was developed in Microsoft® Excel, adopting a UK NHS perspective. Transition probabilities and cost data (year 2004 values) were obtained from published literature or expert opinion. The model incorporated utility values, obtained from members of the public, reflecting the quality of life associated with high-grade glioma. The effects of uncertainty were explored through extensive one-way and probabilistic sensitivity analysis.
Results
Surgery with the implantation of BCNU-W followed by radiotherapy costs £54 500 per additional QALY gained when compared with surgery plus radiotherapy alone. Probabilistic sensitivity analysis shows a <10% probability that BCNU-W would be considered cost effective at a willingness-to-pay threshold of £30 000 per QALY. Although model outputs were sensitive to alterations in several key parameters, the incremental cost effectiveness of the intervention remained above £30 000 per QALY in all analyses.
Conclusion
Compared with usual care for the treatment of newly diagnosed high-grade gliomas, BCNU-W is unlikely to be considered a cost-effective use of healthcare resources when judged by the standards commonly adopted in England and Wales. However, the dreadful prognosis of the condition and the paucity of alternative therapies are additional issues that healthcare commissioners may choose to take into account when considering an adoption decision.








Similar content being viewed by others
Notes
The use of trade names is for product identification purposes only and does not imply endorsement.
References
Souhami RL, Tannock I, Hohenberger P, et al. Oxford textbook of oncology. 2nd ed. Oxford: Oxford University Press, 2001
Behin A, Hoang-Xuan K, Carpentier AF, et al. Primary brain tumours in adults. Lancet 2003; 361 (9354): 323–331
Dinnes J, Cave C, Huang S, et al. The effectiveness and cost-effectiveness of temozolomide for the treatment of recurrent malignant glioma: a rapid and systematic review. Health Technol Assess 2001; 5 (13): 1–73
Berg G, Blomquist E, Cavallin-Stahl E. A systematic overview of radiation therapy effects in brain tumours. Acta Oncol 2003; 42 (5–6): 582–588
Laperriere N, Zuraw L, Cairncross G. Radiotherapy for newly diagnosed malignant glioma in adults: a systematic review. Radiother Oncol 2002 Sep; 64 (3): 259–273
Mitchell P, Ellison DW, Mendelow AD. Surgery for malignant gliomas: mechanistic reasoning and slippery statistics. Lancet Neurol 2005; 4 (7): 413–422
Stewart LA. Chemotherapy in adult high-grade glioma: a systematic review and meta-analysis of individual patient data from 12 randomised trials. Lancet 2002 Mar 23; 359 (9311): 1011–1018
Walker MD, Alexander Jr E, Hunt WE, et al. Evaluation of BCNU and/or radiotherapy in the treatment of anaplastic gliomas: a cooperative clinical trial. JNeurosurg 1978 Sep; 49 (3): 333–343
Solero CL, Monfardini S, Brambilla C, et al. Controlled study with BCNU vs CCNU as adjuvant chemotherapy following surgery plus radiotherapy for glioblastoma multiforme. Cancer Clin Trials 1979; 2 (1): 43–48
Walker MD, Green SB, Byar DP, et al. Randomized comparisons of radiotherapy and nitrosoureas for the treatment of malignant glioma after surgery. N Engl J Med 1980 Dec 4; 303 (23): 1323–1329
Green SB, Byar DP, Walker MD, et al. Comparisons of carmustine, procarbazine, and high-dose methylprednisolone as additions to surgery and radiotherapy for the treatment of malignant glioma. Cancer Treat Rep 1983; 67 (2): 121–132
Chang CH, Horton J, Schoenfeld D, et al. Comparison of postoperative radiotherapy and combined postoperative radiotherapy and chemotherapy in the multidisciplinary management of malignant gliomas: a joint Radiation Therapy Oncology Group and Eastern Cooperative Oncology Group study. Cancer 1983 Sep 15; 52 (6): 997–1007
Nelson DF, Diener-West M, Horton J, et al. Combined modality approach to treatment of malignant gliomas: reevaluation of RTOG 7401/ECOG 1374 with long-term follow-up. A joint study of the Radiation Therapy Oncology Group and the Eastern Cooperative Oncology Group. NCI Monogr 1988; (6): 279–284
Kupersmith MJ, Frohman LP, Choi IS, et al. Visual system toxicity following intra-arterial chemotherapy. Neurology 1988 Feb; 38 (2): 284–289
Bashir R, Hochberg FH, Linggood RM, et al. Pre-irradiation internal carotid artery BCNU in treatment of glioblastoma multiforme. J Neurosurg 1988 Jun; 68 (6): 917–919
Kleinschmidt-DeMasters BK, Geier JM. Pathology of high-dose intraarterial BCNU. Surg Neurol 1989 Jun; 31 (6): 435–443
Yang MB, Tamargo RJ, Brem H. Controlled delivery of 1,3-bis (2-chloroethyl)-l-nitrosourea from ethylene-vinyl acetate copolymer. Cancer Res 1989 Sep 15; 49 (18): 5103–5107
Brem H, Mahaley Jr MS, Vick NA, et al. Interstitial chemotherapy with drug polymer implants for the treatment of recurrent gliomas. J 1991 Mar; 74 (3): 441–446
Brem H, Ewend MG, Piantadosi S, et al. The safety of interstitial chemotherapy with BCNU-loaded polymer followed by radiation therapy in the treatment of newly diagnosed malignant gliomas: phase I trial. J Neurooncol 1995 Nov; 26 (2): 111–123
Garside R, Pitt M, Anderson R, et al. The effectiveness and cost-effectiveness of carmustine implants and temozolomide for the treatment of newly diagnosed high-grade glioma: a systematic review and economic evaluation. Health Technol Assess 2007; 11 (45): 1–242
Westphal M, Hilt DC, Bortey E, et al. A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro Oncol 2003 Apr; 5 (2): 79–88
Westphal M, Ram Z, Riddle V, et al. Gliadel® wafer in initial surgery for malignant glioma: long-term follow-up of a multi-center controlled trial. Acta Neurochir (Wien) 2006 Mar; 148 (3): 269–275
National Institute for Clinical Excellence. Guidance for manufacturers and sponsors [report no. 5]. London: National Institute for Clinical Excellence, 2001
Sonnenberg FA, Beck JR. Markov models in medical decision making: a practical guide. Med Decis Making 1993; 13: 322–338
Barker FG, Prados MD, Chang SM, et al. Radiation response and survival time in patients with glioblastoma multiforme. J Neurosurg 1996 Mar; 84 (3): 442–448
Barnholtz-Sloan JS, Sloan AE, Schwartz AG. Relative survival rates and patterns of diagnosis analyzed by time period for individuals with primary malignant brain tumor, 1973–1997. J Neurosurg 2003 Sep; 99 (3): 458–466
CBTRUS: Central Brain Tumor Registry of the United States. Primary brain tumors in the United States: statistical report 1997–2001. Hinsdale(IL): CBTRUS, 2004 [online]. Available from URL: http://www.cbtms.org/reports/2004-2005/2005reportpdf [Accessed 2007 Nov 23]
US Food and Drug Administration. Approval package: gliadel wafer (20-637/S16). 2003 July 8 [online]. Available from URL: http://www.fda.gov/cder/foi/nda/2003/20-637s016_GliadeLhtm [Accessed 2005 Jul 18]
Chang SM, Parney IF, McDermott M, et al. Perioperative complications and neurological outcomes of first and second craniotomies among patients enrolled in the Glioma Outcome Project. J Neurosurg 2003 Jun; 98 (6): 1175–1181
Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005 Mar 10; 352 (10): 987–996
Collett D. Modelling survival data in medical research. 2nd ed. Boca Raton (FL): Chapman & Hall, 2003
Department of Health. Reference costs 2004. London: Department of Health, 2005
British Medical Association, Royal Pharmaceutical Society of Great Britain. British national formulary no. 49 (March 2005). Wallingford: Pharmaceutical Press, 2005
Guest JF, Ruiz FJ, Russ J, et al. A comparison of the resources used in advanced cancer care between two different strong opioids: an analysis of naturalistic practice in the UK. Curr Med Res Opin 2005; 21: 271–280
National Institute for Health and Clinical Excellence. Guide to the methods of technology appraisal. London: National Institute for Clinical Excellence, 2004
Canadian Coordinating Office for Health Technology Assessment. Guidelines for economic evaluation of pharmaceuticals: Canada. 2nd ed. Ottawa (ON): Canadian Coordinating Office for Health Technology Assessment (CCOHTA), 1997
Gold MR, Siegel JE, Russel LB, et al. Cost-effectiveness in health and medicine. Oxford: Oxford University Press, 1996
NHS Value of Health Panel Project 2005 [online]. Available from URL: http://www.valueofhealth.org/ [Accessed 2005 Nov 2]
Osoba D, Aaronson NK, Muller M, et al. Effect of neurological dysfunction on health-related quality of life in patients with high-grade glioma. J Neurooncol 1997 Sep; 34 (3): 263–278
Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993 Mar 3; 85 (5): 365–376
Osoba D, Aaronson C, Muller NK, et al. The development and psychometric validation of a brain cancer quality-of-life questionnaire for use in combination with general cancer-specific questionnaires. Qual Life Res 1996; 5: 139–150
Doubilet P, Begg CB, Weinstein MC, et al. Probabilistic sensitivity analysis using Monte Carlo simulation: practical approach. Med Decis Making 1985; 5 (2): 157–177
Critchfield GC, Willard KE. Probabilistic analysis of decision trees using Monte Carlo simulation. Med Decis Making 1986 Apr; 6 (2): 85–92
Fenwick E, Claxton K, Sculpher M. Representing uncertainty: the role of cost-effectiveness acceptability curves. Health Econ 2001 Dec; 10 (8): 779–787
Salo J, Niemela A, Joukamaa M, et al. Effect of brain tumour laterality on patients’ perceived quality o’ life. J Neurol Neurosurg Psychiatry 2002 Mar; 72 (3): 373–377
Davies E, Clarke C, Hopkins A. Malignant cerebral glioma: II. Perspectives of patients and relatives on the value of radiotherapy. BMJ 1996 Dec 14; 313 (7071): 1512–1516
Davies E, Hildebrand J. Communication, information and support for adults with malignant cerebral glioma: a systematic literature reveiw. Support Care Cancer 2003; 11: 21–29
Macdonald DR, Cascino TL, Schold SC, et al. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 1990 Jul; 8 (7): 1277–1280
Reference for a preliminary ruling from the Bundesgerichtshof (Germany) in the proceedings brought by Massachusetts Institute of Technology: case C-431/04. European Court of Justice, 2006 May 4. CVRIA© [online]. Available from URL: http:// curia.europa.eu/jurisp/cgi-bin/form.pl?lang=en&Submit-Sub mit&numaff-C431/04 [Accessed 2007 Nov 23]
Cairncross JG, Ueki K, Zlatescu MC, et al. Specific genetic predictors of chemotherapeutic response and survival in patients with anaplastic oligodendrogliomas. J Natl Cancer Inst 1998 Oct 7; 90 (19): 1473–1479
Ino Y, Betensky RA, Zlatescu MC, et al. Molecular subtypes of anaplastic oligodendroglioma: implications for patient management at diagnosis. Clin Cancer Res 2001; 7 (4): 839–845
Rawlins MD, Culyer AJ. National Institute for Clinical Excellence and its value judgments. BMJ 2004 Jul 24; 329 (7459): 224–227
National Institute for Health and Clinical Excellence. Guide to the methods of technology appraisal. London: National Institute for Clinical Excellence, 2004
Acknowledgements
The analysis described in this paper was funded as part of the assessment of carmustine implants for newly diagnosed high-grade gliomas commissioned by the UK NHS Research and Development Health Technology Assessment programme (project number 04/20/01). The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the Department of Health.
The authors have no conflicts of interest that are directly relevant to the content of this study.
The analysis described in this paper was undertaken with expert clinical advice from Professor Michael Brada (Professor of Clinical Oncology, The Royal Marsden Hospital, Surrey); Dr Robin Grant (Consultant Neurologist, Western General Hospital, Edinburgh); Mr Vakis Papanastassiou (Senior Lecturer in Neurosurgery, Southern General Hospital, Glasgow). While their input was invaluable in the conception and execution of the analysis, the conclusions drawn here are solely those of the authors.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Rogers, G., Garside, R., Mealing, S. et al. Carmustine Implants for the Treatment of Newly Diagnosed High-Grade Gliomas. Pharmacoeconomics 26, 33–44 (2008). https://doi.org/10.2165/00019053-200826010-00004
Published:
Issue Date:
DOI: https://doi.org/10.2165/00019053-200826010-00004