Abstract
The literature reporting economic evaluations related to the treatment of Alzheimer’s disease (AD) has developed over the last decade. Most analyses have used economic models to estimate the cost effectiveness of drugs for the treatment of AD. This review considers the range of methods used in the published costeffectiveness literature to model AD progression and the effect of interventions on the progression of AD. The review builds on and updates an earlier systematic review of cost-effectiveness studies on drugs for AD.
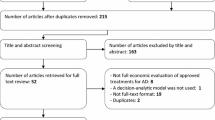
Systematic and rigorous methods were used to search the literature for economic evaluations estimating the cost effectiveness of donepezil, rivastigmine, galantamine or memantine in AD. The literature search covered a wide range of electronic databases (e.g. MEDLINE, EMBASE), and included literature from the inception of databases up to the end of 2005. The search identified 22 published economic evaluations. An outline and brief critical review of the identified studies is provided, and thereafter the methods used to model disease progression were considered in more detail. The review employs recent guidance on good practice in decision-analytic modelling in HTA to critically review the modelling methods used. Using this guidance, the models are assessed against the broad criteria of model structure, data inputs and assessment of uncertainty and inconsistency.
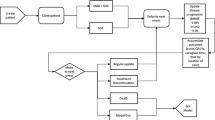
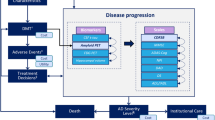
Concerns were noted over the model structure employed in all models. The reliance on cognitive scores to model AD, the progression of the disease, and the effect of treatment on costs and consequences is regarded as a serious limitation in almost all of the studies identified. There are also limitations over the data used to populate published models, especially around the failure of studies to document and establish the basis for the modelling of treatment effects. It is also clear that studies modelling AD progression, and subsequently the cost effectiveness of treatment, have not addressed uncertainty or consistency (internal and/or external) in sufficient detail.
Further research is required on more appropriate methods for the modelling of AD progression. In the meantime, future economic evaluations of treatment need to be more explicit on the methods used to model AD, and the data used to populate models.


Similar content being viewed by others
References
Loveman E, Green C, Kirby J, et al. The clinical and cost effectiveness of donepezil, rivastigmine, galantamine, and memantine for Alzheimer’s disease. Health Technol Assess 2006; 10 (1): 1–176
Birks JS, Harvey R. Donepezil for dementia due to Alzheimer’s disease. The Cochrane Library, Issue 1. Chichester: John Wiley & Sons, Ltd, 2004
Birks J, Grimley EJ, Iakovidou V, et al. Rivastigmine for Alzheimer’s disease. The Cochrane Library, Issue 1. Chichester: John Wiley & Sons, Ltd, 2004
Olin J, Schneider L. Galantamine for Alzheimer’s disease. Cochrane Database Syst Rev 2001; (4): CD001747
National Institute for Health and Clinical Excellence. Appraisal consultation document: donepezil, rivastigmine, galantamine and memantine for the treatment of Alzheimer’s disease [online]. Available from URL: http://guidance.nice.org.uk/ page.aspx?o=245908 [Accessed 2006 Jul 31]
Kirby J, Green C, Loveman E et al. A systematic review of the clinical and cost-effectiveness of memantine in patients with moderately-severe to severe Alzheimer’s disease. Drugs Aging 2006; 23 (3): 227–240
Lanctot KL, Risebrough N, Oh PI. Factors affecting the economic attractiveness of cognitive enhancers in Alzheimer’s disease [abstract]. J Clin Pharmacol 1998; 38: 870
Sobolewski M, Kuzniar J, Splawinski J. Is donepezil cost-effective in the treatment of Alzheimer’s disease? [abstract] Neurobiol Aging 2002; 23 (1): 368
Brooks E Deal L. The effect of rivastigmine on the direct and indirect costs of Alzheimer’s disease [abstract]. Value Health 2000; 3 (2): 79
Antonanzas F, Badenas J, Francois C, et al. Cost effectiveness of memantine in the treatment of moderately severe and severe Alzheimer’s disease in Spain [abstract]. Value Health 2003; 6 (6): 765
Guilhaume C. Cost effectiveness of memantine in the treatment of moderately severe and severe Alzheimer’s disease in Finland. Eur J Neurol 2003 Sep; 10 Suppl. 1: 159–160
Launois R, Guilhaume C, Francois C, et al. Cost effectiveness of memantine in the treatment of moderately severe and severe Alzheimer’s disease in Norway [abstract]. International Conference on Alzheimer’s and Parkinson’s Diseases; 2003 May 8–12; Seville
Stein K. Donepezil in the treatment of mild to moderate senile dementia of the Alzheimer type (SDAT) [report no. 69]. Bristol: NHS Executive South and West, 1997
Stein K. Rivastigmine (Exelon) in the treatment of senile dementia of the Alzheimer type (SDAT) [report no. 89]. Bristol: NHS Executive South and West, 1998
Foster RH, Plosker GL. Donepezil. Pharmacoeconomic implications of therapy. Pharmacoeconomics 1999 Jul; 16 (1): 99–114
Lyseng-Williamson K, Plosker GL. Galantamine: a pharmacoeconomic review of its use in Alzheimer’s disease. Pharmacoeconomics 2002; 20 (13): 919–942
Lamb HM, Goa KL. Rivastigmine: a pharmacoeconomic review of its use in Alzheimer’s disease. Pharmacoeconomics 2001; 1 (3): 303–318
Plosker GL, Lyseng-Williamson KA. Memantine: a pharmacoeconomic review of its use in moderate-to-severe Alzheimer’s disease. Pharmacoeconomics 2005; 23 (2): 193–206
Clegg A, Bryant J, Nicholson T, et al. Clinical and cost-effectiveness of donepezil, rivastigmine and galantamine for Alzheimer’s disease: a rapid and systematic review. Health Technol Assess 2001; 5 (1): 1–137
Wolf son C, Oremus M, Shukla V, et al. Donepezil and rivastigmine in the treatment of Alzheimer’s disease: a best-evidence synthesis of the published data on their efficacy and cost-effectiveness. Clin Ther 2002 Jun; 24 (6): 862–886
Caro J, Salas M, Ward A, et al. Assessing the health and economic impact of galantamine treatment in patients with Alzheimer’s disease in the health care systems of different countries. Drugs Aging 2004; 21 (10): 677–686
Stewart A, Phillips R, Dempsey G. Pharmacotherapy for people with Alzheimer’s disease: a Markov-cycle evaluation of five years’ therapy using donepezil. Int J Geriatr Psychiatry 1998; 13 (7): 445–453
Jonsson L, Lindgren P, Wimo A, et al. The cost-effectiveness of donepezil therapy in Swedish patients with Alzheimer’s disease: a Markov model. Clin Ther 1999; 21 (7): 1230–1240
O’Brien BJ, Goeree R, Hux M, et al. Economic evaluation of donepezil for the treatment of Alzheimer’s disease in Canada. J Am Geriatr Soc 1999; 47 (5): 570–578
Neumann PJ, Hermann RC, Kuntz KM, et al. Cost-effectiveness of donepezil in the treatment of mild or moderate Alzheimer’s disease. Neurology 1999 Jun; 52 (6): 1138–1145
Ikeda S, Yamada Y, Ikegami N. Economic evaluation of donepezil treatment for Alzheimer’s disease in Japan. Dement Geriatr Cogn Disord 2002; 13 (1): 33–39
Fagnani F, Lafuma A, Pechevis M, et al. Donepezil for the treatment of mild to moderate Alzheimer’s disease in France: the economic implications. Dement Geriatr Cogn Disord 2003; 17 (1–2): 5–13
Wimo A, Winblad B, Engedal K, et al. An economic evaluation of donepezil in mild to moderate Alzheimer’s disease: results of a 1-year, double-blind, randomized trial [published erratum appears in Dement Geriatr Cogn Disord 2003; 16 (2): 102]. Dement Geriatr Cogn Disord 2003; 15 (1): 44–54
AD2000 collaborative group. Long-term donepezil treatment in 565 patients with Alzheimer’s disease (AD2000): randomised double-blind trial. Lancet 2004; 363: 2105–2115
Fenn P, Gray A. Estimating long term cost savings from treatment of Alzheimer’s disease: a modelling approach. Pharmacoeconomics 1999; 16 (2): 165–174
Hauber AB, Gnanasakthy A, Snyder EH, et al. Potential savings in the cost of caring for Alzheimer’s disease: treatment with rivastigmine. Pharmacoeconomics 2000; 17 (4): 351–360
Hauber AB, Gnanasakthy A, Mauskopf JA. Savings in the cost of caring for patients with Alzheimer’s disease in Canada: an analysis of treatment with rivastigmine. Clin Ther 2000 Apr; 22 (4): 439–451
Getsios D, Caro JJ, Caro G, et al. Assessment of health economics in Alzheimer’s disease (AHEAD): galantamine treatment in Canada. AHEAD Study Group. Neurology 2001 Sep 25; 57 (6): 972–978
Garfield FB, Getsios D, Caro JJ, et al. Assessment of Health Economics in Alzheimer’s Disease (AHEAD): treatment with galantamine in Sweden. Pharmacoeconomics 2002; 20 (9): 629–637
Caro JJ, Getsios D, Migliaccio-Walle K, et al. Assessment of Health Economics in Alzheimer’s Disease (AHEAD) based on need for fulltime care. Neurology 2001; 57 (6): 964–971
Migliaccio-Walle K, Getsios D, Caro JJ, et al. Economic evaluation of galantamine in the treatment of mild to moderate Alzheimer’s disease in the United States. Clin Ther 2003 Jun; 25 (6): 1806–1825
Ward A, Caro JJ, Getsios D, et al. Assessment of health economics in Alzheimer’s disease (AHEAD): treatment with galantamine in the UK. Int J Geriatr Psychiatry 2003 Aug; 18 (8): 740–747
Caro J, Getsios D, Migliaccio-Walle K, et al. Rational choice of cholinesterase inhibitor for the treatment of Alzheimer. BMC Geriatr 2003; 3 (6)
Green C, Picot J, Loveman E, et al. Modelling the c ost effectiveness of cholinesterase inhibitors in the management of mild to moderately severe Alzheimer’s disease. Pharmacoeconomics 2005; 23 (12): 1271–1282
Francois C, Sintonen H, Sulkava R. The cost-effectiveness of memantine in moderately severe to severe Alzheimer’s disease: a Markov model in Finland. Clin Drug Investig 2004; 27 (7): 373–384
Jones RW, Soininen H, Hager K, et al. A multinational, randomised, 12-week study comparing the effects of donepezil and galantamine in patients with mild to moderate Alzheimer’s disease. Int J Geriatr Psychiatry 2004 Jan; 19 (1): 58–67
Jonsson L. Cost-effectiveness of memantine for moderate to severe Alzheimer’s disease in Sweden. Am J Geriatr Pharmacother 2005; 3 (2): 77–86
Miners AH, Garau M, Fidan D, et al. Comparing estimates of cost effectiveness submitted to the National Institute for Clinical Excellence (NICE) by different organisations: retrospective study. BMJ 2005; 330 (7482): 65
Als-Nielsen B, Chen W, Gluud C, et al. Association of funding and conclusions in randomised dmg trials: a reflection of treatment effect or adverse events? JAMA 2003; 290: 921–928
Lexchin J, Bero LA, Djulbegovic B, et al. Pharmaceutical industry sponsorship and research outcome and quality: systematic review. BMJ 2003; 326: 1167–1170
Drummond MF, O’Brien B, Stoddart GL, et al. Methods for the economic evaluation of health care programmes. 2nd ed. New York: Oxford University Press, 1997
Drummond M, Jefferson TO. Guidelines for authors and peer reviewers of economic submissions to the BMJ. The BMJ Economic Evaluation Working Party. BMJ 1996 Aug 3; 313 (7052): 275–283
Philips Z, Ginnelly L, Sculpher M, et al. A review of guidelines for good practice in decision-analytic modelling in health technology assessment. Health Technol Assess 2004; 8 (36): iii–iv, ix-xi, 1-158
Beck JR, Pauker SG. The Markov process in medical prognosis. Med Decis Making 1983; 3: 419–458
Briggs A, Sculpher M, Claxton K. Decision modelling for health economic evaluation. New York: Oxford University Press, 2006
Rosier M, Anand R, Cicin SA, et al. Efficacy and safety of rivastigmine in patients with Alzheimer’s disease: international randomised controlled trial. BMJ 1999; 318 (7184): 633–640
Corey-Bloom J, Anand R, Veach J. A randomized trial evaluating the efficacy and safety of ENA 713 (rivastigmine tartrate), a new acetylcholinesterase inhibitor, in patients with mild to moderately severe Alzheimer’s disease. Int J Geriatr Psychopharmacol 1998; 1 (2): 55–65
Stern Y, Tang MX, Albert MS, et al. Predicting time to nursing home care and death in individuals with Alzheimer disease. JAMA 1997 Mar 12; 277 (10): 806–812
Reisberg B, Doody R, Stoffler A, et al. Memantine in moderate-to-severe Alzheimer’s disease. N Engl J Med 2003 Apr 3; 348 (14): 1333–1341
Livingston G, Katona C, Roch B, et al. A dependency model for patients with Alzheimer’s disease: its validation and relationship to the costs of care. The LASER-AD Study. Curr Med Res Opin 2004; S7: 1007–1016
Fratiglioni L, Viitanen M, Backman L, et al. Occurrence of dementia in advanced age: the study design of the Kungsholm Project. Neuroepidemiology 1992; 11 Suppl. 1: 29–69
Neumann PJ, Araki SS, Arcelus A, et al. Measuring Alzheimer’s disease progression with transition probabilities: estimates from CERAD. Neurology 2001 Sep 25; 57 (6): 957–964
Mendiondo MS, Ashford JW, Kryscio RJ, et al. Modelling mini mental state examination changes in Alzheimer’s disease. Stat Med 2000 Jun 15; 19 (11–12): 1607–1616
Morris JC, Heyman A, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part I: clinical and neuropsychological assessment of Alzheimer’s disease. Neurology 1989 Sep; 39 (9): 1159–1165
Bowie P, Branton T, Holmes J. Should the Mini Mental State Examination be used to monitor dementia treatments? Lancet 1999 Oct 30; 354 (9189): 1527–1528
Davey RJ, Jamieson S. The validity of using the Mini Mental State Examination in NICE dementia guidelines. J Neurol Neurosurg Psychiatry 2004 Feb; 75 (2): 343–344
Tombaugh TN, Mcintyre NJ. The Mini-Mental-State-Examination: a comprehensive review. J Am Geriatr Soc 1992; 40 (9): 922–935
Wolstenholme J, Fenn P, Gray A, et al. Estimating the relationship between disease progression and cost of care in dementia. Br J Psychiatry 2002; 181: 36–42
Clark CM, Sheppard L, Fillenbaum G, et al. Variability in Annual Mini-Mental State Examination score in patients with probable Alzheimer’s disease: a clinical perspective of data from the Consortium to Establish a Registry for Alzheimer’s Disease. Arch Neurol 1999; 56: 857–862
Neumann PJ, Kuntz KM, Leon J, et al. Health utilities in Alzheimer’s disease: a cross-sectional study of patients and caregivers. Med Care 1999 Jan; 37 (1): 27–32
Andersen K, Nielsen H, Lolk A, et al. Incidence of very mild to severe dementia and Alzheimer’s disease in Denmark: the Odense Study. Neurology 1999 Jan 1; 52 (1): 85–90
Andersen CK, Wittrup-Jensen KU, Lolk A, et al. Ability to perform activities of daily living is the main factor affecting quality of life in patients with dementia. Health Qual Life Outcomes 2004; 2 (52)
Caro JJ, Salas M, Ward A, et al. Economic analysis of galantamine, a cholinesterase inhibitor, in the treatment of patients with mild to moderate Alzheimer’s disease in the Netherlands. Dement Geriatr Cogn Disord 2002; 14 (2): 84–89
Knapp M, Wilkinson D, Wigglesworth R. The economic consequences of Alzheimer’s disease in the context of new drug developments. Int J Geriatr Psychiatry 1998 Feb; 13 (8): 531–543
Acknowledgements
The review builds on earlier work by the author and colleagues at the Southampton Health Technology Assessments Centre (SHTAC), University of Southampton, Southampton, UK, and he is grateful to colleagues there for their general help and assistance in the background work for this review. Special thanks go to Elizabeth Payne, Alison Price and Liz Hodson for their help with the literature search, and to Dr Jo Picot for her comments on the cost-effectiveness literature. The author has no conflicts of interest that are directly relevant to the content of this review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Green, C. Modelling Disease Progression in Alzheimer’s Disease. Pharmacoeconomics 25, 735–750 (2007). https://doi.org/10.2165/00019053-200725090-00003
Published:
Issue Date:
DOI: https://doi.org/10.2165/00019053-200725090-00003