Abstract
Objective: To estimate the cost effectiveness (from the UK NHS and personal social services perspective) of the cholinesterase inhibitors donepezil, rivastigmine and galantamine compared with usual care in the treatment of mild to moderately severe Alzheimer’s disease. Patients had a mean age of 74 years, a mean disease duration of 1 year and a mean Alzheimer’s disease assessment scale-cognitive subscale score of 24.
Methods: A pharmacoeconomic model was used to predict long-term outcomes over a 5-year time horizon and to estimate the cost effectiveness of cholinesterase inhibitors for the management of Alzheimer’s disease. The model structure is informed by a systematic review of the literature on the clinical and cost effectiveness of cholinesterase inhibitors and a review of the literature on the costs and outcomes associated with treatment for Alzheimer’s disease. The main outcome measure used was the cost per quality-adjusted life-year (QALY) gained. All healthcare costs (excluding cholinesterase inhibitor costs) were indexed to £ (2003 values). Drug costs are 2005 values. Multivariate probabilistic sensitivity analysis and scenario analysis were undertaken to assess uncertainty in the results.
Results: The clinical benefits on cognition from treatment with cholinesterase inhibitors resulted in an incremental cost per QALY gained ranging from £53 780 to £74 735, over 5 years (vs usual care). Uncertainty analysis suggests that the probability of any of these treatments having an incremental cost per QALY of <£30 000 is <21%. The key determinants of cost effectiveness were the effectiveness of treatment, the mean treatment cost and the cost savings associated with an expected delay in disease progression.
Conclusions: Results presented in this paper suggest that the use of cholinesterase inhibitors may not be a cost-effective use of NHS resources. Guidance from the National Institute for Health and Clinical Effectiveness (NICE) in the UK on their judgements surrounding the acceptability of technologies as an effective use of resources, indicates there would need to be special reasons for accepting cholinesterase inhibitors as a cost-effective use of NHS resources.






Similar content being viewed by others
Notes
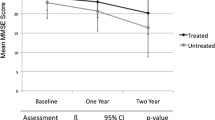
ADAS-cog measures orientation, memory, language and praxis on a scale of 0–70, with higher scores indicating greater impairment.
MMSE includes 11 questions on orientation, memory, concentration, language and praxis, and uses a scale of 0–30, with a higher score indicating less impairment.
References
AD2000 Collaborative Group. Long-term donepezil treatment in 565 patients with Alzheimer’s disease (AD2000): randomised double-blind trial. Lancet 2004; 363: 2105–15
Wimo A, Winblad B, Engedal K, et al. An economic evaluation of donepezil in mild to moderate Alzheimer’s disease: results of a 1-year, double-blind, randomized trial. Dement Geriatr Cogn Disord 2003; 15 (1): 44–54
Ward A, Caro JJ, Getsios D, et al. Assessment of Health Economics in Alzheimer’s Disease (AHEAD): treatment with galantamine in the UK. Int J Geriatr Psychiatry 2003; 18 (8): 740–7
Migliaccio-Walle K, Getsios D, Caro JJ, et al. Economic evaluation of galantamine in the treatment of mild to moderate Alzheimer’s disease in the United States. Clin Ther 2003; 25 (6): 1806–25
Garfield FB, Getsios D, Caro JJ, et al. Assessment of Health Economics in Alzheimer’s Disease (AHEAD): treatment with galantamine in Sweden. Pharmacoeconomics 2002; 20 (9): 629–37
Getsios D, Caro JJ, Caro G, et al. Assessment of Health Economics in Alzheimer’s Disease (AHEAD): galantamine treatment in Canada. AHEAD Study Group. Neurology 2001; 57 (6): 972–8
Ikeda S, Yamada Y, Ikegami N. Economic evaluation of donepezil treatment for Alzheimer’s disease in Japan. Dement Geriatr Cogn Disord 2002; 13 (1): 33–9
Hauber AB, Gnanasakthy A, Mauskopf JA. Savings in the cost of caring for patients with Alzheimer’s disease in Canada: an analysis of treatment with rivastigmine. Clin Ther 2000; 22 (4): 439–51
Jonsson L, Lindgren P, Wimo A, et al. The cost-effectiveness of donepezil therapy in Swedish patients with Alzheimer’s disease: a Markov model. Clin Ther 1999; 21 (7): 1230–40
Neumann PJ, Hermann RC, Kuntz KM, et al. Cost-effectiveness of donepezil in the treatment of mild or moderate Alzheimer’s disease. Neurology 1999; 52 (6): 1138–45
O’Brien BJ, Goeree R, Hux M, et al. Economic evaluation of donepezil for the treatment of Alzheimer’s disease in Canada. J Am Geriatr Soc 1999; 47 (5): 570–8
Stewart A, Phillips R, Dempsey G. Pharmacotherapy for people with Alzheimer’s disease: a Markov-cycle evaluation of five years’ therapy using donepezil. Int J Geriatr Psychiatry 1998; 13 (7): 445–53
Caro JJ, Salas M, Ward A, et al. Economic analysis of galantamine, a cholinesterase inhibitor, in the treatment of patients with mild to moderate Alzheimer’s disease in the Netherlands. Dement Geriatr Cognit Disord 2002; 14 (2): 84–9
Hauber AB, Gnanasakthy A, Snyder EH, et al. Potential savings in the cost of caring for Alzheimer’s disease: treatment with rivastigmine. Pharmacoeconomics 2000; 17 (4): 351–60
National Institute for Clinical Excellence. Guidance on the use of donepezil, rivastigmine and galantamine for the treatment of Alzheimer’s disease. Technology Appraisal Guidance (No. 19). London: NICE, 2001 [online] Available from URL: http:// www.nice.org.uk/pdf/ALZHEIMER full-guidance.pdf [Accessed 2005 Nov 25]
Loveman E, Green C, Kirby J, et al. The clinical and cost effectiveness of donepezil, rivastigmine, galantamine, and memantine for Alzheimer’s disease. Health Technol Assess. In press 2005 December; 19 (9) in press
Bowie P, Branton T, Holmes J. Should the Mini Mental State Examination be used to monitor dementia treatments? Lancet 1999; 354 (9189): 1527–8
Davey RJ, Jamieson S. The validity of using the mini mental state examination in NICE dementia guidelines. J Neurol Neurosurg Psychiatry 2004; 75 (2): 343–4
Tombaugh TN, Mcintyre NJ. The mini-mental-state-examination: a comprehensive review. J Am Geriatr Soc 1992; 40 (9): 922–35
Neumann PJ, Araki SS, Arcelus A, et al. Measuring Alzheimer’s disease progression with transition probabilities: estimates from CERAD. Neurology 2001; 57 (6): 957–64
Mendiondo MS, Ashford JW, Kryscio RJ, et al. Modelling mini mental state examination changes in Alzheimer’s disease. Stat Med 2000; 19 (11-12): 1607–16
Caro JJ, Getsios D, Migliaccio-Walle K, et al. Assessment of health economics in Alzheimer’s disease (AHEAD) based on need for full-time care. Neurology 2001; 57 (6): 964–71
Stern Y, Tang MX, Albert MS, et al. Predicting time to nursing home care and death in individuals with Alzheimer disease. JAMA 1997; 277 (10): 806–12
Martin DC, Miller JK, Kapoor W, et al. A controlled study of survival with dementia. Arch Neurol 1987; 44 (11): 1122–6
Burns A, Forstl H. The Institute of Psychiatry Alzheimer’s disease cohort: part I. Clinical observations. Int J Geriatr Psychiatry 1996; 11: 309–20
Wolstenholme J, Fenn P, Gray A, et al. Estimating the relationship between disease progression and cost of care in dementia. Br J Psychiatry 2002; 181: 36–42
Dodge HH, Shen C, Pandav R, et al. Functional transitions and active life expectancy associated with Alzheimer disease. Arch Neurol 2003; 60 (2): 253–9
Alzheimer’s Society. Appraisal of drugs for Alzheimer’s disease: submission to NICE. London: 2004
Wilkinson D, Stave C, Keohane D, et al. The role of general practitioners in the diagnosis and treatment of Alzheimer’s disease: a multinational survey. J Int Med Res 2004; 32 (2): 149–59
Netten A, Curtis L. Unit costs of health and social care 2003. Canterbury (UK): University of Kent, 2004
British National Formulary. 49 (March). London: British Medical Association and the Royal Pharmaceutical Society of Great Britain, 2005
Kavanagh S, Knapp M. Costs and cognitive disability: modelling the underlying associations. Br J Psychiatry 2002; 180: 120–5
Netten A, Darton R, Bebbington A, et al. Residential and nursing home care of elderly people with cognitive impairment: prevalence, mortality and costs. Aging Ment Health 2001; 5 (1): 14–22
Martin J, Meltzer H, Elliot D. The prevalence of disability among adults: OPCS surveys of disability in Great Britain. Report 1. London: HMSO, 1988
Burns A, Rossor M, Hecker J, et al. The effects of donepezil in Alzheimer’s disease: results from a multinational trial. Dementia Geriatr Cogn Disord 1999; 10 (3): 237–44
Gauthier S, Feldman H, Hecker J, et al. Functional, cognitive and behavioral effects of donepezil in patients with moderate Alzheimer’s disease. Curr Med Res Opin 2002; 18 (6): 347–54
Greenberg SM, Tennis MK, Brown LB, et al. Donepezil therapy in clinical practice: a randomized crossover study. Arch Neurol 2000; 57: 94–9
Holmes C, Wilkinson D, Dean C, et al. The efficacy of donepezil in the treatment of neuropsychiatric symptoms in Alzheimer’s disease. Neurology 2004; 63: 214–9
Homma A, Takeda M, Imai Y, et al. Clinical efficacy and safety of donepezil on cognitive and global function in patients with Alzheimer’s disease: a 24-week, multicenter, double-blind, placebo-controlled study in Japan. Dement Geriatr Cogn Disord 2000; 11 (6): 299–313
Krishnan KR, Charles HC, Doraiswamy PM, et al. Randomized, placebo-controlled trial of the effects of donepezil on neuronal markers and hippocampal volumes in Alzheimer’s disease. Am J Psychiatry 2003 Nov; 160 (11): 2003–11
Mohs RC, Doody RS, Morris JC, et al. A 1-year, placebocontrolled preservation of function survival study of donepezil in AD patients. Neurology 2001 Aug 14; 57 (3): 481–8
Nunez M, Hasselbalch S, Henn R, et al. Donepezil-treated Alzheimer’s disease patients with apparent initial cognitive decline demonstrate significant benefits when therapy is continued: results from a randomised, placebo-controlled trial [poster]. The Second Annual Dementia Congress; 2003 Sep 12-14; Washington, DC
Rogers SL, Friedhoff LT, Apter JT, et al. The efficacy and safety of donepezil in patients with Alzheimer’s disease: results of a US multicentre, randomized, double-blind, placebocontrolled trial. Dementia 1996; 7 (6): 293–303
Rogers SL, Doody RS, Mohs RC, et al. Donepezil improves cognition and global function in Alzheimer disease: a 15-week, double-blind, placebo-controlled study. Arch Intern Med 1998; 158 (9): 1021–31
Rogers SL, Farlow MR, Doody RS, et al. A 24-week, doubleblind, placebo-controlled trial of donepezil in patients with Alzheimer’s disease: donepezil Study Group. Neurology 1998 Jan; 50 (1): 136–45
Winblad B, Engedal K, Soininen H, et al. A 1-year, randomized, placebo-controlled study of donepezil in patients with mild to moderate AD. Neurology 2001 Aug 14; 57 (3): 489–95
Agid Y, Dubois B, Anand R, et al. Efficacy and tolerability of rivastigmine in patients with dementia of the Alzheimer type. Curr Ther Res Clin Exp 1998; 59 (12): 837–45
Corey-Bloom J, Anand R, Veach J. A randomized trial evaluating the efficacy and safety of ENA 713 (rivastigmine tartrate), a new acetylcholinesterase inhibitor, in patients with mild to moderately severe Alzheimer’s disease. Int J Geriatr Psychopharmacol 1998; 1 (2): 55–65
Forette F, Anand R, Gharabawi G. A phase II study in patients with Alzheimer’s disease to assess the preliminary efficacy and maximum tolerated dose of rivastigmine. Fur J Neurol 1999; 6 (4): 423–9
Rosler M, Anand R, Cicin SA, et al. Efficacy and safety of rivastigmine in patients with Alzheimer’s disease: international randomised controlled trial. BMJ 1999; 318 (7184): 633–40
Raskind MA, Peskind ER, Wessel T, et al. Galantamine in AD: a 6-month randomised, placebo-controlled trial with a 6-month extension. Neurology 2000; 54: 2261–8
Rockwood K, Mintzer J, Truyen L, et al. Effects of a flexible galantamine dose in Alzheimer’s disease: a randomised, controlled trial. J Neurol Neurosurg Psychiatry 2001 Nov; 71 (5): 589–95
Tariot P, Solomon PR, Morris J, et al. A 5-month, randomised, placebo-controlled trial of galantamine in AD. Neurology 2000; 54: 2269–76
Wilcock GK, Lilienfeld S, Gaens E. Efficacy and safety of galantamine in patients with mild to moderate Alzheimer’s disease: multicentre randomised controlled trial. BMJ 2000; 321 (7274): 1445–9
Wilkinson D, Lilienfeld S, Truyen L. Galantamine improves activities of daily living in patients with Alzheimer’s disease: a 3-month placebo-controlled study [abstract]. Proceedings of the Sixth International Stockholm/Springfield Symposium on Advances in Alzheimer Therapy; 2000 Apr 5-8; Stockholm
Wilkinson D, Murray J. Galantamine: a randomized, doubleblind, dose comparison in patients with Alzheimer’s disease. Int J Geriatr Psychiatry 2001 Sep; 16 (9): 852–7
Fuschillo C, La Pia S, Campana F, et al. Cognitive deficits in Alzheimer’s disease: treatment with acetylcholinesterase inhibitor agents. Arch Gerontol Geriatr Suppl 2001; 7: 151–8
Jones RW, Soininen H, Hager K, et al. A multinational, randomised, 12-week study comparing the effects of donepezil and galantamine in patients with mild to moderate Alzheimer’s disease. Int J Geriatr Psychiatry 2004 Jan; 19 (1): 58–67
Wilkinson DG, Passmore AP, Bullock R, et al. A multinational, randomised, 12-week, comparative study of donepezil and rivastigmine in patients with mild to moderate Alzheimer’s disease. Int J Clin Pract 2002 Jul; 56 (6): 441–6
Wolfson C, Moride Y, Perrault A, et al. Drug treatments for Alzheimers’s disease: 1. A comparative analysis of clinical trials. Ottawa: Canadian Coordinating Office for Health Technology Assessment (CCOHTA), 2000
Birks JS, Harvey R. Donepezil for dementia due to Alzheimer’s disease. The Cochrane Library, Issue 1, 2004. Chichester, UK: John Wiley & Sons Ltd, 2004
Birks J, Grimley EJ, Iakovidou V, et al. Rivastigmine for Alzheimer’s disease. The Cochrane Library, Issue 1, 2004. Chichester, UK: John Wiley & Sons Ltd, 2004
Olin J, Schneider L. Galantamine for Alzheimer. Cochrane Database of Systematic Reviews 2001; (4)
Gray A, Fenn P. Alzheimer’s disease: the burden of the illness in England. Health Trends 1993; 25 (1): 31–7
O’shea E, O’Reilly S. The economic and social cost of dementia in Ireland. Int J Geriatr Psychiatry 2000; 15 (3): 208–18
Alzheimer’s Society, London. Paying home care fees. Alzheimer’s Society Information Sheet (468). 2004 [online] Available from URL: http://www.alzheimers.org.uk/Caring for someone_with_dementia/PDF/468_PayingForCare.pdf [Accessed 2005 Nov 25]
Whitehouse P. Measurements of quality of life in dementia. In: Wimo A, Jonsson B, Karlsson G, et al., editors. Health economics of dementia. Chichester (UK): John Wiley & Sons, 1998
Neumann PJ. Measuring QALYS in dementia. In: Wimo A, Jonsson B, Karlsson G, et al., editors. Health economics of dementia. Chichester (UK): John Wiley & Sons Ltd, 1998
Williams A. The measurement and valuation of health: a chronicle. Discussion paper 136. York (UK): University of York (Centre for Health Economics), 1995
Neumann PJ, Sandberg EA, Araki SS, et al. A comparison of HUI2 and HUI3 utility scores in Alzheimer’s disease. Med Decis Making 2000; 20 (4): 413–22
Torrance GW, Feeny DH, Furlon WJ, et al. Multi-attribute preference functions for a comprehensive health status classification system: Health Utilities Index Mark 2. Med Care 1996; 24: 702–22
National Institute for Clinical Excellence. Guidance for manufacturers and sponsors. London: National Institute for Clinical Excellence, 2001
Burns A, Forstl H. The Institute of Psychiatry Alzheimer’s disease cohort: part II. Clinicopathological observations. Int J Geriatr Psychiatry 1996; 11: 321–7
Lopez OL, Becker JT, Wisniewski S, et al. Cholinesterase inhibitor treatment alters natural history of Alzheimer’s disease. J Neurol Neurosurg Psychiatry 2002; 72: 310–4
Hui JS, Wilson RS, Bennett DA, et al. Rate of cognitive decline and mortality in Alzheimer’s disease. Neurology 2003; 61 (10): 1356–61
National Institute for Clinical Excellence. Guide to the methods of technology appraisal. London: National Institute for Clinical Excellence, 2004 Apr
National Institute for Clinical Excellence. Appraisal consultation document: donepezil, rivastigmine, galantamine and memantine for the treatment of Alzheimer’s disease. London: NICE, 2005 Feb [online]. Available from URL: http://www. nice.org.uk [Accessed 2005 Nov 25]
Acknowledgements
The study was completed as part of a review funded by the UK NHS R&D Health Technology Assessment Programme, and commissioned on behalf of the NICE. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Department of Health.
Colin Green developed the cost-effectiveness model and undertook cost-effectiveness analysis. All authors contributed to the review of clinical and cost-effectivenss literature, and all authors contributed to the drafting and preparation of the paper.
All authors declare that they have no competing or conflicts of interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Green, C., Picot, J., Loveman, E. et al. Modelling the cost effectiveness of cholinesterase inhibitors in the management of mild to moderately severe Alzheimer’s disease. Pharmacoeconomics 23, 1271–1282 (2005). https://doi.org/10.2165/00019053-200523120-00010
Published:
Issue Date:
DOI: https://doi.org/10.2165/00019053-200523120-00010