Abstract
Objective: To evaluate the cost utility of imatinib compared with interferon (IFN)-α or hydroxycarbamide (hydroxyurea) for first-line treatment of chronic myeloid leukaemia.
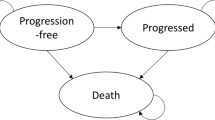
Design and Setting: A cost-utility (Markov) model within the setting of the UK NHS and viewed from a health system perspective was adopted. Transition probabilities and relative risks were estimated from published literature. Costs of drug treatment, outpatient care, bone marrow biopsies, radiography, blood transfusions and inpatient care were obtained from the British National Formulary and local hospital databases. Costs (£, year 2001–03 values) were discounted at 6%. Quality-of-life (QOL) data were obtained from the published literature and discounted at 1.5%. The main outcome measure was cost per QALY gained. Extensive one-way sensitivity analyses were performed along with probabilistic (stochastic) analysis.
Results: The incremental cost-effectiveness ratio (ICER) of imatinib, compared with IFNα, was £26 180 per QALY gained (one-way sensitivity analyses ranged from £19 449 to £51 870) and compared with hydroxycarbamide was £86 934 per QALY (one-way sensitivity analyses ranged from £69 701 to £147 095) [£1 = $US1.691 = €1.535 as at 31 December 2002].
Based on the probabilistic sensitivity analysis, 50% of the ICERs for imatinib, compared with IFNα, fell below a threshold of approximately £31 000 per QALY gained. Fifty percent of ICERs for imatinib, compared with hydroxycarbamide, fell below approximately £95 000 per QALY gained.
Conclusions: This model suggests, given its underlying data and assumptions, that imatinib may be moderately cost effective when compared with IFNα but considerably less cost effective when compared with hydroxycarbamide. There are, however, many uncertainties due to the lack of long-term data.











Similar content being viewed by others
References
Kumar P. Clinical medicine. 4th ed. Clin Med 2001; 4: 425–32
Sawyers C. Chronic myeloid leukemia. N Engl J Med 1999; 340: 1330–40
Hemandez-Boluda JC, Cervantes F, Alvarez A, et al. Singleagent therapy with oral mercaptopurine for nonlymphoid blast crisis of chronic myeloid leukemia. Ann Hematol 2001; 80: 516–20
Delannoy A, Kluin-Nelemans JC, Louwagie A, et al. Interferon alfa versus chemotherapy for chronic myeloid leukemia: a meta-analysis of seven randomized trials. J Nall Cancer Inst 1997; 89 (21): 1616–20
Kalidas M, Kantarjian H, Talpaz M. Chronic myelogenous leukemia. JAMA 2001; 286: 895–8S
Garside R, Round A, Dalziel K, et al. The effectiveness and cost-effectiveness of imatinib in chronic myeloid leukaemia: a systematic review. Health Technol Assess 2002; 6 (33): 1–162
Talpaz M, Kantarjian HM, McCredie KB, et al. Clinical investigation of human alpha interferon in chronic myelogenous leukemia. Blood 1987; 69: 1280–8
Hehlmann R, Willer A, Heimpel H, et al. Randomized studies with interferon in chronic myelogenous leukemia (CML) and comparative molecular aspects. Leukemia 1997; 11 Suppl. 3: 506–11
Long-term follow-up of the Italian trial of interferon-alpha versus conventional chemotherapy in chronic myeloid leukemia. The Italian Cooperative Study Group on Chronic Myeloid Leukemia. Blood 1998; 92: 1541–8
Novartis Pharmaceuticals UK Ltd. Appraisal of Glivec for 1st line treatment of chronic myeloid leukaemia. Chamberley: Novartis Pharma AG, 2003 (data on file)
O’Brien SG, Guilhot F, Larson RA, et al., the IRIS Investigators. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med 2003; 348: 994–1004
Benelux CML Study Group. Randomized study on hydroxyurea alone versus hydroxyurea combined with low-dose interferon alpha 2b for chronic myeloid leukemia. Blood 1998; 91: 2713–21
Dalziel K, Round A, Stein K, Garside R, et al. The effectiveness and cost effectiveness of imatinib for first line treatment of chronic myeloid leukaemia in chronic phase. Monograph 70. London: National Institute for Clinical Excellence, 2003 Oct
Kantarjian HM, Smith TL, O’Brien S, et al. Prolonged survival in chronic myelogenous leukemia after cytogenetic response to interferon-alpha therapy. Ann Intern Med 1995; 122: 254–61
Bonifazi F. Chronic myeloid leukemia and interferon-alpha: a study of complete cytogenetic responders. Blood 2001; 98: 3074–81
Kantarjian HM, Talpaz M, O’Brien S, et al. Imatinib mesylate for Philadelphia chromosome-positive, chronic-phase myeloid leukemia after failure of interferon-alpha: follow-up results. Clin Cancer Res 2002; 8: 2177–87
Bonifazi F, De Vivo A, Rosti G, et al. Testing Sokal’s and the new prognostic score for chronic myeloid leukaemia treated with alpha-interferon. Br J Haematol 2000; 111: 587–95
British National Formulary 44. London: British Medical Association and Royal Pharmaceutical Association of Great Britian, September 2002
Allan NC, Richards SM, Shepherd PC. UK Medical Research Council randomised, multicentre trial of interferon-alpha nl for chronic myeloid leukaemia: improved survival irrespective of cytogenetic response. The UK Medical Research Council’s Working Parties for Therapeutic Trials in Adult Leukaemia. Lancet 1995; 345: 1392–7
Kattan MW, Inoue Y, Giles FJ, et al. Cost-effectiveness of interferon-alpha and conventional chemotherapy in chronic myelogenous leukemia. Ann Intern Med 1996; 125: 541–8
Gordois A, Scuffham P, Warren E, et al. Cost utility analysis of imatinib mesylate for the treatment of advanced stage chronic myeloid leukaemia. Br J Cancer 2003; 89: 634–40
Freund MG. Combination of chemotherapy and interferon alfa-2b in the treatment of chronic myelogenous leukemia. Semin Hematol 1993; 30: 11–3
Drummond M, McGuire A. Economic evaluation in health care. Oxford: Oxford University Press, 2001
Gambacorti-Passerini C, Gunby R, Piazza R, et al. Molecular mechanisms of resistance to imatinib in Philadelphia-chromosome-positive leukaemias. Lancet Oncol 2003; 4: 75–85
Liberato NL, Quaglini S, Barosi G. Cost-effectiveness of interferon alfa in chronic myelogenous leukemia. J Clin Oncol 1997; 15: 2673–82
Messori A. A cost-effectiveness of interferon in chronic myeloid leukaemia: analysis of four clinical studies. Ann Oncol 1998; 9: 389–96
Raftery J. Faster access to modern treatments? Analysis of guidance on health technologies. BMJ 2001; 323: 1300–3
European Agency for the Evaluation of Medicinal Products (EMEA). Procedure for orphan medicinal product designation: general principles. London: EMEA, 2002
Radford I. Imatinib. Novartis. Curr Opin Investig Drugs 2002; 3: 492–9
Acknowledgements
Kim Dalziel contributed to the design of the study, obtained parameter estimates, implemented the cost-effectiveness model and drafted the report.
Ken Stein and Ali Round contributed to the design of the study, reviewed the cost-effectiveness model and drafted the report.
Ruth Garside contributed to the design of the study, obtained parameter estimates and drafted the report.
Source of funding: UK NHS Research and Development (R&D) Health Technology Assessment Programme (Grant number 01/34/01). The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dalziel, K., Round, A., Garside, R. et al. Cost effectiveness of imatinib compared with interferon-α or hydroxycarbamide for first-line treatment of chronic myeloid leukaemia. Pharmacoeconomics 23, 515–526 (2005). https://doi.org/10.2165/00019053-200523050-00010
Published:
Issue Date:
DOI: https://doi.org/10.2165/00019053-200523050-00010