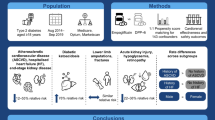
Summary
Abstract
Eptifibatide (Integrilin®) is a selective inhibitor of platelet glycoprotein (GP) IIb/ IIIa receptors used as adjunctive therapy for patients undergoing percutaneous coronary intervention (PCI) and for patients with acute coronary syndromes (ACS), particularly those requiring PCI. Most economic analyses of eptifibatide have incorporated clinical and healthcare resource use data from either the ESPRIT (Enhanced Suppression of the Platelet IIb/IIIa Receptor with Integrilin® Therapy) study in low-to moderate-risk patients undergoing selective PCI with stent implantation or the PURSUIT (Platelet Glycoprotein IIb/IIIa in Unstable Angina: Receptor Suppression Using Integrilin® Therapy) trial in patients with ACS. Eptifibatide achieved statistically significant reductions in combined endpoints of death and ischaemic complications in both of these large multicentre clinical trials, in which patients were randomised to receive intravenous eptifibatide or placebo as adjunctive therapy to heparin and aspirin (plus a thienopyridine in ESPRIT).
In US economic analyses using ESPRIT trial data, approximately 40% and 70% of the acquisition cost of eptifibatide was offset by reduced medical resource consumption during the initial hospitalisation period and over a 1-year period, respectively. Eptifibatide was associated with a favourable cost-effectiveness ratio of $US1407 (year 2000 costs) per life-year gained (LYG) in a retrospective US cost-effectiveness analysis that incorporated data from the ESPRIT trial and modelled life expectancy using a large cardiovascular database.
Several cost-effectiveness analyses used prospectively collected data from the PURSUIT trial and modelled survival projections using similar methods. These analyses, conducted in the US, Canada and Western Europe, also showed favourable results ($US3761–$US18 774 per LYG; various years of costing). Cost-utility ratios reported in US analyses varied somewhat, but remained <$US20 000 per quality-adjusted life-year gained (1996 values) when clinical efficacy data were derived from the US cohort of PURSUIT.
Conclusion: Significant clinical benefits have been demonstrated with eptifibatide as adjunctive therapy in patients undergoing selective PCI with stent implantation in the ESPRIT trial and in patients with ACS in the PURSUIT trial. Pharmacoeconomic analyses using data from either ESPRIT or PURSUIT have demonstrated favourable cost-effectiveness ratios for both indications in various countries. ESPRIT-based results from the limited number of available economic analyses are particularly favourable. The cost-effectiveness of eptifibatide in ACS (i.e. PURSUIT-based results) may be further improved by targeting the drug for patients in whom catheterisation and PCI are planned, although further analyses are required to confirm this.
Percutaneous Coronary Intervention (PCI) and Acute Coronary Syndromes (ACS)
Percutaneous coronary intervention (PCI) refers to all forms of percutaneous mechanical revascularisation and is a relatively common intervention in patients with coronary artery disease, with more than 1 million angioplasty procedures conducted in the US each year. Acute coronary syndromes (ACS), for the purposes of this review, comprise unstable angina and non-ST-segment elevation myocardial infarction (NSTEMI). Each year, approximately 2–2.5 million patients are hospitalised worldwide because of unstable angina or NSTEMI, and at least half of these are in the US.
In-hospital costs for patients undergoing PCI and planned stent implantation were estimated at $US10 430 per patient (without a glycoprotein [GP] IIb/IIIa receptor antagonist; year 2000 costs) in a large retrospective US cost analysis, although both higher and lower estimates of costs associated with PCI have also been reported. Total discounted 10-year medical costs were estimated at $US50 254 per patient (1997 values) in a study in 4319 patients with unstable angina who underwent an initial cardiac catheterisation at a US medical centre between 1986 and 1997. More than 40% of costs were incurred during the acute phase of therapy (which did not include GP IIb/IIIa receptor antagonists).
Clinical Profile of Eptifibatide
GP IIb/IIIa receptor antagonists such as eptifibatide inhibit the final common pathway for platelet aggregation. Therefore, these drugs can play an important role in preventing thrombus formation in coronary arteries, including that caused by mechanical disruption of an atherosclerotic plaque (e.g. during PCI) or via chemically mediated activation of platelet GP IIb/IIIa receptors (e.g. by thromboxane A2 or adenosine diphosphate). Eptifibatide is a selective GP IIb/IIIa receptor antagonist indicated for patients with coronary artery disease who require selective (nonurgent) or urgent PCI and for those with ACS (many of whom require urgent PCI). The drug is administered intravenously and is used as an adjunct to therapy with heparin and aspirin (acetylsalicylic acid).
The clinical efficacy of eptifibatide in patients with low- to moderate-risk coronary artery disease undergoing selective PCI with stent implantation was demonstrated in the ESPRIT (Enhanced Suppression of the Platelet IIb/IIIa Receptor with Integrilin® Therapy) trial. The study excluded higher-risk patients such as those with ongoing chest pain. Patients were randomised to receive eptifibatide (n = 1040) or placebo (n = 1024), in addition to heparin, aspirin and a thienopyridine (clopidogrel or ticlopidine). Eptifibatide was administered as a bolus of 180 μg/kg followed immediately by a continuous infusion of 2 μg/kg/min (for 18–24 hours or until discharge from hospital, whichever occurred first), and a second bolus dose of 180 ug/kg administered 10 minutes after the first bolus. Results of the primary efficacy analysis showed that, at 48 hours after randomisation, 6.6% of eptifibatide recipients and 10.5% of placebo recipients (p = 0.0015) had either died, experienced a myocardial infarction, underwent urgent target vessel revascularisation (UTVR), or required open-label eptifibatide because of thrombotic complications. An important secondary combined endpoint of death, myocardial infarction and UTVR at 30 days was similarly reduced with eptifibatide versus placebo (6.8% vs 10.5%, p = 0.0034).
The clinical efficacy of eptifibatide in patients with ACS was demonstrated in the PURSUIT (Platelet Glycoprotein IIb/IIIa in Unstable Angina: Receptor Suppression Using Integrilin® Therapy) trial. In this large, double-blind, multicentre study, analysis of efficacy included 9461 patients who were randomised to receive eptifibatide (180 μg/kg bolus followed by a continuous infusion of 2 μg/kg/min until hospital discharge for up to a duration of 72 hours, or up to 96 hours in those who underwent PCI) or placebo. A large majority of patients received concomitant heparin and aspirin, although such therapy was not mandated in this trial. For the primary endpoint of death or myocardial infarction at 30 days after randomisation, eptifibatide was associated with a significant benefit compared with placebo (14.2% vs 15.7% of patients, p = 0.04), and this effect was observed early during therapy. Approximately 13% of patients required early PCI within 72 hours of randomisation, and eptifibatide appeared to be particularly beneficial for this subgroup. However, results of subgroup analyses with inadequate statistical power must be interpreted with caution.
The most important adverse events associated with eptifibatide are major and minor bleeding (including the need for transfusion). In general, the incidence of bleeding and the need for transfusion was greater with eptifibatide than placebo in large multicentre trials. However, the incidence of bleeding complications varied widely between the studies, possibly because of differences in risk factors and eptifibatide dosage protocols. Stroke was reported in 0.7% of eptifibatide recipients and a similar proportion of placebo recipients (0.8%) in the PURSUIT study. Stroke was rare in the ESPRIT trial and haemorrhagic stroke was rare in both multicentre studies.
Pharmacoeconomic Analyses of Eptifibatide
In Patients Undergoing PCI: Prospective economic analyses of the ESPRIT trial in patients undergoing selective PCI with stent implantation have not been conducted. However, a detailed retrospective cost analysis and a briefly reported cost-effectiveness analysis have been conducted in the US using actual medical resource consumption from the ESPRIT study where possible.
Results of the US cost analysis showed that total medical costs associated with the initial hospitalisation were 2.8% higher among eptifibatide than placebo recipients ($US10 722 vs $US10 430 per patient; p < 0.001) [year 2000 costs]. Approximately 40% of the $US495 per patient acquisition cost of eptifibatide was offset by reduced medical resource consumption during the index hospitalisation.
The US cost-effectiveness analysis, which examined the incremental costs and benefits of eptifibatide as an adjunct to standard therapy versus standard therapy alone, used 1-year follow-up data from the ESPRIT study and modelled life expectancy using the Duke Cardiovascular Database. In addition to basic life-expectancy projections, the effect of a nonfatal myocardial infarction on subsequent survival was also incorporated into the model. Results showed that the mean incremental cost of eptifibatide was $US146 per patient ($US12 844 vs $US12 697; year 2000 costs) and life expectancy (discounted at 3%) was projected to increase by 0.104 years, thus providing a favourable cost-effectiveness ratio of $US1407 per life-year gained (LYG). (Minor calculation discrepancies are presumably due to rounding.) Approximately 70% of the acquisition cost of eptifibatide was therefore offset by reduced medical resource consumption during the first year. Cost-effectiveness ratios remained below $US2275 per LYG in the sensitivity analysis, even when the prognostic significance of a nonfatal myocardial infarction was eliminated. In addition, a Canadian cost-effectiveness analysis using data from ESPRIT and a hospital cost database reported that eptifibatide was dominant over standard therapy (i.e. less costly and more effective); however, further details are lacking in this brief report.
A number of retrospective and prospective economic analyses have been conducted comparing eptifibatide with abciximab as adjunctive therapy in patients undergoing PCI. Study methodology was not uniform across these analyses, all of which were conducted in the US. In general, results showed that eptifibatide was associated with lower acquisition costs and similar or lower total medical costs or charges per patient, while clinical outcomes appeared to be similar between the two GP IIb/IIIa receptor antagonists.
Two prospectively conducted comparisons have been performed. In a randomised trial in 320 patients undergoing selective PCI, total inpatient costs were the primary endpoint. Median total in-hospital costs per patient (1999/2000 values) were $US7207 with eptifibatide compared with $US8268 for abciximab (p < 0.01), and clinical efficacy was deemed to be similar between treatment groups. The difference in total medical costs was primarily because of the lower acquisition cost for eptifibatide versus abciximab. The other prospective study was not randomised and did not include a formal economic analysis per se. Nevertheless, the acquisition cost of eptifibatide was lower than that of abciximab and there was little difference between groups in terms of major cost drivers (e.g. length of hospital stay, major adverse cardiovascular events) when used in patients undergoing PCI for a variety of indications.
Analyses in Patients with ACS: A number of pharmacoeconomic analyses of eptifibatide in ACS (with or without PCI) have been conducted prospectively using data from the PURSUIT trial. In these analyses, medical resource consumption data were collected for the US, Canadian, Western European and other specific cohorts of the PURSUIT trial, and unit costs for the geographical region of interest were then applied to determine total medical costs per patient. In all of these studies, the acquisition cost of eptifibatide was at least partly offset by a reduced need for medical intervention. Total medical costs, measured over a 6-month period in the majority of analyses, were 1.1–6.8% higher among eptifibatide than placebo recipients, although UK-specific resource consumption was lower in the eptifibatide group.
Several of the studies were cost-effectiveness analyses, in which long-term survival was estimated using modelled projections made from 6-month results of the PURSUIT trial, in most cases for the cohort of patients from the particular geographical region of the economic substudy. Cost-effectiveness ratios, which reflected incremental costs and benefits of eptifibatide as an adjunct to standard therapy versus standard therapy alone, were favourable for eptifibatide and ranged from approximately $US3761–$US18 774 per LYG (various years of costing). Values not reported in US dollars were converted using mid-year exchange rates for the year of costing or, if not provided, for the year of study publication. One US analysis also reported a cost-utility ratio of $US19 693 per quality-adjusted life-year (QALY) gained (1996 costs), although a somewhat less favourable cost-utility ratio ($US42 469 per QALY; year of costing not provided) was reported by other US investigators who used clinical results of the entire study population of PURSUIT (rather than the more favourable results of the US cohort) to estimate long-term survival.






Similar content being viewed by others
Notes
Tradename is included for identification purposes only and does not imply product endorsement.
References
Goa KL, Noble S. Eptifibatide: a review of its use in patients with acute coronary syndromes and/or undergoing percutaneous coronary intervention. Drugs 1999 Mar; 57 (3): 439–62
Integrilin (eptifibatide) injection prescribing information. San Francisco (CA): COR Therapeutics Inc., 2001
Braunwald E, Amman EM, Beasley JW, et al. ACC/AHA guideline update for the management of patients with unstable angina and non-ST-segment elevation myocardial infarction. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on the Management of Patients with Unstable Angina) [online]. Available from URL: http://www.ace.org [Accessed 2002 Jun 4]
Braunwald E, Amman EM, Beasley JW, et al. ACC/AHA guideline update for the management of patients with unstable angina and non-ST-segment elevation myocardial infarction2002: summary article. Circulation 2002 Oct 1, 1893–900
Dery JP, O’Shea JC, Tcheng JE. Eptifibatide in percutaneous coronary intervention. A review. Minerva Cardioangiol 2002 Oct; 50 (5): 531–46
Inhibition of platelet glycoprotein IIb/IIIa with eptifibatide in patients with acute coronary syndromes. PURSUIT Trial Investigators. N Engl J Med 1998; 339: 436–43
Novel dosing regimen of eptifibatide in planned coronary stent implantation (ESPRIT): a randomised, placebo-controlled trial. ESPRIT Investigators. Lancet 2000 Dee 16; 356 (9247): 2037–44
Popma JJ, Ohman EM, Weitz J, et al. Antithrombotic therapy in patients undergoing percutaneous coronary intervention. Chest 2001; 119 Suppl.: 321S–36S
Belli G, Ellis SG, Topol EJ. Stenting for ischemic heart disease. Prog Cardiovasc Dis 1997; 40: 159–82
Truong KM, Amankwa K, Kucukarslan S. Platelet glycoprotein IIb/IIIa-receptor inhibitors in patients with acute coronary syndromes or undergoing percutaneous coronary interven tions: a review. Clin Ther 2001 Aug; 23 (8): 1145–65; discussion 1129
Bhatt DL, Topol EJ. Current role of platelet glycoprotein IIb/ IIIa inhibitors in acute coronary syndromes. JAMA 2000 Sep 27; 284 (12): 1549–58
Yusuf S, Flather M, Pogue J, et al. Variations between countries in invasive cardiac procedures and outcomes in patients with suspected unstable angina or myocardial infarction without initial ST elevation: OASIS Registry Investigators. Lancet 1998; 352: 507–14
American Heart Association. 2001 Heart and Stroke Statistical Update. Heart attack and angina statistics [online]. Available from URL: http://www.americanheart.org [Accessed 2002 Jun 4]
Cannon CP. Glycoprotein IIb/IIIa inhibitors in unstable angina and non-ST segment elevation myocardial infarction. Coron Artery Dis 1999; 10 (8): 561–6
McElwee NE, Johnson ER. Potential economic impact of glycoprotein IIb-IIIa inhibitors in improving outcomes of patients with acute ischemic coronary syndromes. Am J Cardiol 1997; 80 (4 A): 39B–43B
Eisenstein EL, Shaw LK, Anstrom KJ, et al. Assessing the clinical and economic burden of coronary artery disease: 1986–1998. Med Care 2001 Aug; 39 (8): 824–35
Cohen DJ, O’ Shea JC, Pacchiana CM, et al. In-hospital costs of coronary stent implantation with and without eptifibatide (the ESPRIT trial). Enhanced Suppression of the Platelet IIb/IIIa Receptor with Integrilin. Am J Cardiol 2002 Jan 1; 89 (1): 61–4
McKay RG, Boden WE. Small peptide GP IIb/IIIa receptor inhibitors as upstream therapy in non-ST-segment elevation acute coronary syndromes: results of the PURSUIT, PRISM, PRISM-PLUS, TACTICS, and PARAGON trials. Curr Opin Cardiol 2001 Nov; 16 (6): 364–9
Lefkovits J, Plow EF, Topol EJ. Platelet glycoprotein IIb/IIIa receptors in cardiovascular medicine. N Engl J Med 1995; 332: 1553–9
Jarvis B, Simpson K. Clopidogrel: a review of its use in the prevention of atherothrombosis. Drugs 2000 Aug; 60 (2): 347–77
Lincoff AM, Califf RM, Topol EJ. Platelet glycoprotein IIb/IIIa receptor blockade in coronary artery disease. J Am Coll Cardiol 2000 Apr; 35 (5): 1103–15
Randomised placebo-controlled trial of effect of eptifibatide on complications of percutaneous coronary intervention: IMPACT-II. The IMPACT Investigators. Lancet 1997; 349: 1422–8
Phillips DR, Teng W, Arfsten A, et al. Effect of Cat+ on GP IIb-IIIa interactions with integrilin: enhanced GP IIb-IIIa binding and inhibition of platelet aggregation by reductions in the concentration of ionized calcium in plasma anticoagulated with citrate. Circulation 1997; 96: 1488–94
O’Shea JC, Buller CE, Cantor WJ, et al. Long-term efficacy of platelet glycoprotein IIb/IIIa integrin blockade with eptifibatide in coronary s tent intervention. JAMA 2002 Feb 6; 287 (5): 618–21
O’Shea JC, Halley GE, Greenberg S, et al. Platelet glycoprotein IIb/IIIa integrin blockade with eptifibatide in coronary stent intervention: the ESPRIT trial: a randomized controlled trial. JAMA 2001 May 16; 285 (19): 2468–73
Blankenship JC, Tasissa G, O’Shea JC, et al. Effect of glycoprotein IIb/IIIa receptor inhibition on angiographic complications during percutaneous coronary intervention in the ESPIRIT trial. J Am Coll Cardiol 2001 Sep; 38: 653–8
Cantor WJ, Hellkamp AS, O’ Shea JC, et al. Bailout platelet GP IIb/IIIa inhibition in coronary stent implantation: observations from the ESPRIT trial [abstract no. 885-3]. J Am Coll Cardiol 2001 Feb; 37 Suppl. A: 84A
Chandler B, O’Shea JC, Greenberg S, et al. Robust benefit of platelet GP IIb/IIIa inhibition with eptifibatide in patients undergoing stent PCI with hot or cold presentation. J Am Coll Cardiol 2001 Feb; 37 Suppl. A: 76A–7A
Fernandes LS, Tcheng JE, Kleiman NS, et al. Eptifibatide is as effective in women as in men: lessons from the ESPRIT trial [abstract no. 3790]. Circulation 2000 Oct 31; 102 Suppl.: 785
Gibson CM, Cohen DJ, Cohen EA, et al. Effect of eptifibatide on coronary flow reserve following coronary stent implantation (an ESPRIT substudy). Enhanced Suppression of the Platelet IIb/IIIa Receptor with Integrilin Therapy. Am J Cardiol 2001 Jun 1; 87 (11): 1293–5
Labinaz M, Madan M, McGuire DK, et al. Effect of eptifibatide, a glycoprotein IIb/IIIa antagonist, among diabetic patients following coronary stenting: results from ESPRIT [abstract no. 3215]. Circulation 2000 Oct 31; 102 Suppl.: 665
O’Shea JC, Lorenz TJ, Joseph D, et al. Robust benefit of glycoprotein IIb/IIIa inhibition with eptifibatide in patients undergoing stent percutaneous coronary intervention with pos itive cardiac markers at baseline [abstract no. TCT-30]. Am J Cardiol 2000 Oct 16; 86: 13i–4i
O’Shea JC, Cohen EA, Buller CEH, et al. Predictors of improvement in clinical outcomes of non-acute coronary stenting in the Esprit trial. Circulation 2000 Oct 31; 102 Suppl.: II–665
O’Shea JC, Mann T, Hellkamp A, et al. Fewer bleeding complications with comparable efficacy with the transradial approach in coronary artery stenting: an analysis of the ESPRIT trial [abstract no. 1123-1129]. J Am Coll Cardiol 2001 Feb; 37 Suppl. A: 33
Hillegass WB, Newman AR, Raco DL. Economic issues in glycoprotein IIb/IIIa receptor therapy. Am Heart J 1999 Jul; 138 (1 Pt 2): S24–32
Dyke CM, Bhatia D, Lorenz TJ, et al. Immediate coronary artery bypass surgery after platelet inhibition with eptifibatide: results from PURSUIT. Platelet Glycoprotein IIb/IIIa in Un stable Angina: Receptor Suppression Using Integrelin Therapy. Ann Thorac Surg 2000 Sep; 70 (3): 866–71; discussion 871-2
Akkerhuis KM, Deckers JW, Boersma E, et al. Geographic variability in outcomes within an international trial of glycoprotein IIb/IIIa inhibition in patients with acute coronary syn dromes. Results from PURSUIT. Fur Heart J 2000 Mar; 21 (5): 371–81
Boersma E, Pieper KS, Steyerberg EW, et al. Predictors of outcome in patients with acute coronary syndromes without persistent ST-segment elevation: results from an international trial of 9461 patients. Circulation 2000 Jun 6; 101: 2557–67
Cohen MG, Pacchiana CM, Corbalan R, et al. Variation in patient management and outcomes for acute coronary syndromes in Latin America and North America: results from the Platelet IIb/IIIa in Unstable Angina: Receptor Suppression Using Integrilin Therapy (PURSUIT) trial. Am Heart J 2001 Mar; 141: 391–401
Greenbaum AB, Harrington RA, Hudson MP, et al. Therapeutic value of eptifibatide at community hospitals transferring patients to tertiary referral centers early after admission for acute coronary syndromes. J Am Coll Cardiol 2001; 37 (2): 492–8
Hasdai D, Holmes Jr DR, Criger DA, et al. Cigarette smoking status and outcome among patients with acute coronary syndromes without persistent ST-segment elevation: effect of inhibition of platelet glycoprotein IIb/IIIa with eptifibatide. PURSUIT trial investigators. Am Heart J 2000 Mar; 139 (3): 454–60
Kleiman NS, Lincoff AM, Flaker GC, et al. Early percutaneous coronary intervention, platelet inhibition with eptifibatide, and clinical outcomes in patients with acute coronary syndromes. Circulation 2000; 101 (7): 751–7
Labinaz M, Kilaru R, Pieper K, et al. Outcomes of patients with acute coronary syndromes and prior coronary artery bypass grafting: results from the platelet glycoprotein IIb/IIIa in unsta ble angina: receptor suppression using integrilin therapy (PURSUIT) trial. Circulation 2002 Jan 22; 105 (3): 322–7
Lauer MA, Houghtaling PL, Peterson JG, et al. Attenuation of rebound ischemia after discontinuation of heparin therapy by glycoprotein IIb/IIIa inhibition with eptifibatide in patients with acute coronary syndromes: observations from the platelet IIb/IIIa in unstable angina: receptor suppression using integrilin therapy (PURSUIT) trial. Circulation 2001 Dec 4; 104 (23): 2772–7
Lincoff AM, Harrington RA, Califf RM, et al. Management of patients with acute coronary syndromes in the United States by platelet glycoprotein IIb/IIIa inhibition: insights from the Platelet Glycoprotein IIb/IIIa in Unstable Angina. Receptor Suppression Using Integrilin Therapy (PURSUIT) trial. Circulation 2000; 102 (10): 1093–100
Mahaffey KW, Harrington RA, Simoons ML, et al. Stroke in patients with acute coronary syndromes: incidence and outcomes in the Platelet Glycoprotein IIb/IIIa in Unstable Angina Receptor Suppression Using Integrilin Therapy (PURSUIT) trial. PURSUIT Investigators. Circulation 1999 May 11; 99 (18): 2371–7
Marso SP, Bhatt DL, Roe MT, et al. Enhanced efficacy of eptifibatide administration in patients with acute coronary syndrome requiring in-hospital coronary artery bypass graft ing. PURSUIT Investigators. Circulation 2000 Dec 12; 102 (24): 2952–8
McClure MW, Berkowitz SD, Sparapani R, et al. Clinical significance of thrombocytopenia during a non-ST-elevation acute coronary syndrome: the platelet glycoprotein IIb/IIIa in unstable angina: receptor suppression using integrilin therapy (PURSUIT) trial experience. Circulation 1999 Jun 8; 99 (22): 2892–900
Peterson JG, Topol EJ, Roe MT, et al. Prognostic importance of concomitant heparin with eptifibatide in acute coronary syndromes. PURSUIT Investigators. Platelet Glycoprotein IIb/IIIa in Unstable Angina: Receptor Suppression Using Integrilin Therapy. Am J Cardiol 2001 Mar 1; 87 (5): 532–6
Akkerhuis M, Boersma E, Harrington RA, et al. Eptifibatide protects against adverse cardiac complications both before and during percutaneous intervention in patients with acute coronary syndromes without ST-elevation [abstract no. 1068-53]. J Am Coll Cardiol 1999 Feb; 33 Suppl. A: 40A
Berdan LG, Lorenz TJ, Hochman IS. Eptifibatide reduces the incidence of coronary events in patients with non-ST-elevation acute coronary syndromes undergoing early cardiac catheteri zation and revascularization regardless of patient gender [abstract no. 1037-104]. J Am Coll Cardiol 2000 Feb; 35 Suppl. A: 343A (plus poster)
Harrington RA, Lincoff MA, Berdan LG, et al. Maintenance of clinical benefit at six-months in patients treated with the platelet glycoprotein IIb/IIIa inhibitor eptifibatide versus placebo during an acute ischemic coronary event [abstract no. 1886]. Circulation 1998; 98: I–359
Alexander JH, Sparapani RA, Mahaffey KW, et al. Eptifibatide reduces the size and incidence of myocardial infarction in patients with non-ST-elevation acute coronary syndromes [ab stract no. 1032-133]. J Am Coll Cardiol 1999; 33 Suppl. A: 331A
Cohen DJ, Cosgrove RS, Berezin RH, et al. Cost-effectiveness of eptifibatide in patients undergoing planned coronary stenting: results from the ESPRIT trial [abstract no. 893153]. Circulation 2001 Oct 23; 104 Suppl. II (17):11–386
Data on file, Schering ACS, Kenilworth, NJ, USA. 2003
Brown RE, Henderson RA, Koster D, et al. Cost effectiveness of eptifibatide in acute coronary syndromes; an economic analysis of Western European patients enrolled in the PUR SUIT trial. Platelet Ha/IIb in Unstable Angina: Receptor Suppression Using Integrilin Therapy. Fur Heart J 2002 Jan; 23 (1): 50–8
Mark DB, Harrington RA, Lincoff AM, et al. Cost-effectiveness of platelet glycoprotein IIb/IIIa inhibition with eptifibatide in patients with non-ST-elevation acute coronary syndromes. Circulation 2000 Feb 1; 101 (4): 366–71
Brown R, Armstrong P. Cost effectiveness in Canada of eptifibatide treatment for acute coronary syndrome patients using PURSUIT subgroup analysis. Can. J. Cardiol. 2003 Feb; 19 (2): 161–6
Sridhar K, Gwadry F, Kidwai B, et al. Decision analysis model of abciximab, eptifibatide or standard therapy in elective stent placement: a Canadian perspective. Value Health 2001; 4: 108
PRICE Investigators. Comparative 30-day economic and clinical outcomes of platelet glycoprotein IIb/IIIa inhibitor use during elective percutaneous coronary intervention: Prairie ReoPro versus Integrilin Cost Evaluation (PRICE) trial. Am Heart J 2001 Mar; 141 (3): 402–9
Lage MJ, Barber BL, McCollam PL, et al. Impact of abciximab versus eptifibatide on length of hospital stay for PCI patients. Catheter Cardiovasc Interv 2001; 53 (3): 296–303
McCollam PL, Luzadis R. Hospital charges in common percutaneous coronary intervention patients receiving abciximab vs eptifibatide [absract no. 189]. Pharmacotherapy 2001 Oct; 21: 1284
Rose L, Hoying M, Przytulski B. A drug use evaluation of abciximab and eptifibatide in a community hospital. PT 2000; 25 (7): 360–6
Wong DH. Comparison of eptifibatide and abciximab with decision analysis. Am J Health Syst Pharm 2001 Aug 1; 58 (15): 1432–6
Schweiger MJ, Changezi HU, Naglieri-Prescod D, et al. Openlabel, sequential comparison of eptifibatide with abciximab for patients undergoing percutaneous coronary intervention. Clin Ther 2003; 25: 225–34
Henderson RA, Brown R. The costs of routine eptifibatide use in acute coronary syndromes in Western Europe: an economic substudy of the PURSUIT trial. Fur Heart J Suppls 1999 Aug; 1 Suppl. N: N35–41
Villar FA, Botella FA. An economic evaluation of eptifibatide [in Spanish]. Rev Esp Cardiol 2001 Feb; 54 (2): 169–74
Brown RE, Hutton J, Henderson R. Cost-effectiveness of eptifibatide in the UK based on PURSUIT trial [abstract no. PCV9]. Value Health 2001 Nov-2001 31; 4: 491
Kent DM, Ruthazer R, Beshansky JR, et al. Effectiveness and cost-effectiveness of eptifibatide in individual patients with acute cardiac ischemia: the importance of risk stratification [abstract no. CV2]. Value Health 2000; 3: 295
Szucs TD, Schwenkglenks M, Berger K, et al. Costs and costeffectiveness of eptifibatide in the treatment of coronary ischemic syndromes. An analysis based on the PURSUIT study [in German]. Z Kardiol 2003 Mar; 92 (3): 236–44
Bank of Canada - Exchange rates results [online]. Available from URL: http://www.bank-banque-canada.ca [Accessed 2002 Jun 20]
Shahriar J, Shaw JW, Malone D. Cost-effectiveness analysis of abciximab, eptifibatide, and tirofiban in patients with coronary syndromes [abstract no. PCV21 plus poster]. Value Health 2001; 4: 103
Cupples ME, Dempster M. Improving quality of life for patients with angina pectoris: a team approach to disease management. Dis Manage Health Outcomes 2001; 9 (9): 473–81
Bliven BD, Green CP, Sperms JA. Review of available instruments and methods for assessing quality of life in anti-anginal trials. Drugs Aging 1998 Oct; 13: 311–20
Gandjour A, Lauterbach KW. Review of quality-of-life evaluations in patients with angina pectoris. Pharmacoeconomics 1999 Aug; 16 (2): 141–52
Thompson SG, Barber JA. How should cost data in pragmatic randomised trials be analysed? BMJ 2000 Apr 29; 320: 1197–200
Zhou X-H, Melfi CA, Hui SL. Methods for comparing cost data. Ann Intern Med 1997; 127 (8 Pt 2): 752–6
Laskey WK, Williams DO, Vlachos HA, et al. Changes in the practice of percutaneous coronary intervention: a comparison of enrollment waves in the National Heart, Lung, and Blood Institute (NHLBI) Dynamic Registry. Am J Cardiol 2001; 87: 964–9
Goldman L, Garber AM, Grover SA, et al. Task force 6. Cost effectiveness of assessment and management of risk factors. J Am Coll Cardiol 1996; 27 (5): 964–1047
Lindholm L, Hallgren C-G, Boman K, et al. Cost-effectiveness analysis with defined budget: how to distribute resources for the prevention of cardiovascular disease? Health Policy 1999; 48: 155–70
Chapman RH, Stone PW, Sandberg EA, et al. A comprehensive league table of cost-utility ratios and a sub-table of ‘panelworthy’ studies. Med Decis Making 2000; 20: 451–67
Mason J, Drummond M, Torrance G. Some guidelines on the use of cost effectiveness league tables. BMJ 1993 Feb 27; 306: 570–2
Drummond ME, O’Brien BJ, Stoddart GL, et al. Presentation and use of economic evaluation results (chapter 9). In: Methods for the economic evaluation of health care programmes. 2nd ed. Oxford: Oxford University Press, 1997: 265–92
Drummond M, Torrance G, Mason J. Cost-effectiveness league tables: more harm than good? Soc Sci Med 1993; 37 (1): 33–40
Laupacis A, Feeny D, Detsky AS, et al. How attractive does a new technology have to be to warrant adoption and utilization? Tentative guidelines for using clinical and economic evaluations. Can Med Assoc J 1992; 146 (4): 473–81
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Plosker, G.L., Ibbotson, T. Eptifibatide. Pharmacoeconomic 21, 885–912 (2003). https://doi.org/10.2165/00019053-200321120-00005
Published:
Issue Date:
DOI: https://doi.org/10.2165/00019053-200321120-00005