Abstract
Objective: To estimate the economic value of pharmacological treatment of type 2 diabetes mellitus in overweight and obese patients using orlistat in addition to standard diabetes therapy (i.e., a sulphonlyurea, metformin or insulin) and weight management strategies as compared with standard diabetes therapy and weight management strategies alone in a US-based healthcare setting. The perspective of the study was from the viewpoint of a US healthcare provider.
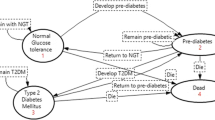
Design and setting: Markov state transition model simulating diabetes-related complications and mortality for a period of 11 years. Patients were modelled to continue orlistat therapy for a 52-week period, assuming a 3-year period of weight regain where after 3 years bodyweight would match that of the placebo group. The impact of orlistat on glycosylated haemoglobin (HbA1c) values was evaluated directly using data from four randomised, placebo-controlled, 1-year trials of orlistat in overweight or obese adults with type 2 diabetes who also received standard diabetes pharmacotherapy and intensive lifestyle modification. Incidence rates of micro- and macrovascular complications associated with type 2 diabetes and the estimated relative reduction in incidence rates associated with a decrease in mean updated HbA1C values were derived from the United Kingdom Prospective Diabetes Study (UKPDS) estimates for a reference population of male patients, 52 years of age.
US cost estimates were derived from published sources and presented in 2001 US dollars. Discounting of 3% was applied. Probabilistic sensitivity analysis was applied to evaluate the robustness of the results of the persistence of the effect of orlistat after treatment.
Main outcome measures: Average costs and event-free life-years gained during the 11-year period expressed as the incremental costs divided by the incremental gain in life expectancy.
Results: Treatment with orlistat, 120mg three times daily, increased event-free life expectancy by 0.13 years over an 11-year period. Average treatment costs were estimated to be $US19 987 in the orlistat group compared with $US18 865 in the group that received diabetes medication and weight management alone. This translated into a cost-effectiveness ratio of $US8327 per event-free life-year gained.
Conclusion: Adding orlistat as a pharmacological treatment to conventional diabetes and weight management approaches seems to be a cost-effective treatment option for overweight and obese patients with type 2 diabetes.







Similar content being viewed by others
Notes
The use of tradenames is for product identification purposes only and does not imply endorsement.
References
Harris MI, Flegal KM, Cowie CC, et al. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U.S. adults. The Third National Health and Nutrition Examination Survey, 1988–1994 [see comments]. Diabetes Care 1998; 21: 518–24
Gaster B, Hirsch IB. The effects of improved glycemic control on complications in type 2 diabetes. Arch Intern Med 1998; 158: 134–40
Brown JB, Pedula KL, Bakst AW. The progressive cost of complications in type 2 diabetes mellitus. Arch Intern Med 1999; 159: 1873–80
American Diabetes Association. Economic consequences of diabetes mellitus in the U.S. in 1997 [see comments]. Diabetes Care 1998; 21: 296–309
Colditz GA, Willett WC, Rotnitzky A, et al. Weight gain as a risk factor for clinical diabetes mellitus in women [see comments]. Ann Intern Med 1995; 122: 481–6
Levy E, Levy P, Le Pen C, et al. The economic cost of obesity: the French situation. Int J Obes Relat Metab Disord 1995; 19: 788–92
Segal L, Carter R, Zimmet P. The cost of obesity: the Australian perspective. Pharmacoeconomics 1994; 5: 45–52
American Diabetes Association. Standards of medical care for patients with diabetes mellitus. Diabetes Care 2002; 25: 213–29
Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002; 346: 393–403
Tuomilehto J, Lindstrom J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance [see comments]. N Engl J Med 2001; 344: 1343–50
Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Pro spective Diabetes Study (UKPDS) Group [see comments] [erratum appears in Lancet 1999 Aug 14; 354 (9178): 602]. Lancet 1998; 352: 837–53
UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group [see comments]. [erratum appears in Lancet 1998 Nov 7; 352 (9139): 1557]. Lancet 1998; 352: 854–65
Gray A, Raikou M, McGuire A, et al. Cost effectiveness of an intensive blood glucose control policy in patients with type 2 diabetes: economic analysis alongside randomised controlled trial (UKPDS 41). United Kingdom Prospective Diabetes Study Group. BMJ 2000; 320: 1373–8
DeFronzo RA. Pharmacologic therapy for type 2 diabetes mellitus. Ann Intern Med 1999; 131: 281–303
Yanovski SZ, Yanovski JA. Obesity. N Engl J Med 2002; 346: 591–602
Lindgarde F. The effect of orlistat on body weight and coronary heart disease risk profile in obese patients: the Swedish Multimorbidity Study [see comments]. J Intern Med 2000; 248: 245–54
Lamotte M, Annemans L, Lefever A, et al. A health economic model to assess the long-term effects and cost-effectiveness of orlistat in obese type 2 diabetic patients. Diabetes Care 2002; 25: 303–8
Hollander PA, Elbein SC, Hirsch IB, et al. Role of orlistat in the treatment of obese patients with type 2 diabetes: a 1-year randomized double-blind study. Diabetes Care 1998; 21: 1288–94
Hanefeld M, Sachse G. The effects of orlistat on body weight and glycaemic control in overweight patients with type 2 diabetes: a randomized, placebo-controlled trial. Diabetes Obes Metab 2002; 4: 415–23
Kelley DE, Bray GA, Pi-Sunyer FX, et al. Clinical efficacy of orlistat therapy in overweight and obese patients with insulin-treated type 2 diabetes: a 1-year randomized controlled trial. Diabetes Care 2002; 25: 1033–41
Miles JM, Leiter L, Hollander PA, et al. Effect of orlistat in overweight and obese patients with type 2 diabetes treated with metformin. Diabetes Care 2002; 25: 1123–8
Sonnenberg FA, Beck JR. Markov models in medical decision making: a practical guide. Med Decis Making 1993; 13: 322–38
National Institute for Clinical Excellence. Guidance on the use of orlistat for the treatment of obesity in adults. Technology appraisal guidance document No. 22, 1–15. 2001. London: National Institute for Clinical Excellence, 2001
Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study [see comments]. BMJ 2000; 321: 405–12
O’Brien JA, Shomphe LA, Kavanagh PL, et al. Direct medical costs of complications resulting from type 2 diabetes in the U.S. Diabetes Care 1998; 21: 1122–8
Four costliest outpatient procedures. Hosp Health Netw 1998; 72: 32–3
IMS Health. The National Prescription Audit™: October–December 1999. Plymouth Meeting (PA): IMS Health, 1999
Cruickshank JK. Glycaemia and vascular effects of type 2 diabetes: UKPDS is not a cohort study and analysis is misleading. BMJ 2001; 322: 1246
McCormack J, Greenhalgh T. Seeing what you want to see in randomised controlled trials: versions and perversions of UKPDS data. United Kingdom prospective diabetes study [see comments]. BMJ 2000; 320: 1720–3
Gold MR, Siegel JE, Russell LB, et al. Cost-effectiveness in health and medicine. New York: Oxford University Press, 1996
Davidson MH, Hauptman J, DiGirolamo M, et al. Weight control and risk factor reduction in obese subjects treated for 2 years with orlistat: a randomized controlled trial [see comments] [erratum appears in JAMA 1999 Apr 7;281(13):1174]. JAMA 1999; 281: 235–42
Fenwick E, Claxton K, Sculpher MI. Representing uncertainty: the role of cost-effectiveness acceptability curves. Health Econ 2001; 10: 779–87
Mittendorfer B, Ostlund RE Jr, Patterson BW, et al. Orlistat inhibits dietary cholesterol absorption. Obes Res 2001; 9: 599–604
Jerant AF, Azari R, Nesbitt TS. Reducing the cost of frequent hospital admissions for congestive heart failure: a randomized trial of a home telecare intervention. Med Care 2001; 39: 1234–45
Caro JJ, Klittich WS, Raggio G, et al. Economic assessment of troglitazone as an adjunct to sulfonylurea therapy in the treatment of type 2 diabetes. Clin Ther 2000; 22: 116–27
Clark Jr CM. The burden of chronic hyperglycemia. Diabetes Care 1998; 21 Suppl. 3: C32–4
Stevens RJ, Kothari V, Adler AI, et al. The UKPDS risk engine: a model for the risk of coronary heart disease in Type II diabetes (UKPDS 56) [see comments]. Clin Sci 2001; 101: 671–9
Doll HA, Petersen SE, Stewart-Brown SL. Obesity and physical and emotional well-being: associations between body mass index, chronic illness, and the physical and mental components of the SF-36 questionnaire. Obes Res 2000; 8: 160–70
Fine IT, Colditz GA, Coakley EH, et al. A prospective study of weight change and health-related quality of life in women. JAMA 1998; 282: 2136–42
Fontaine KR, Cheskin LJ, Barofsky I. Health-related quality of life in obese persons seeking treatment. J Fam Pract 1996; 43: 265–70
Katz DA, McHorney CA, Atkinson RL. Impact of obesity on health-related quality of life in patients with chronic illness. J Gen Intern Med 2000; 15: 789–96
Lean ME, Han TS, Seidell JC. Impairment of health and quality of life using new US federal guidelines for the identification of obesity. Arch Intern Med 1999; 159: 837–43
Wing RR, Koeske R, Epstein LH, et al. Long-term effects of modest weight loss in type II diabetic patients. Arch Intern Med 1987; 147: 1749–53
Hauptman J. Orlistat: selective inhibition of caloric absorption can affect long-term body weight. Endcr J Uk 2000; 13: 201–6
Rossner S, Sjostrom L, Noack R, et al. Weight loss, weight maintenance, and improved cardiovascular risk factors after 2 years treatment with orlistat for obesity. European Orlistat Obesity Study Group. Obes Res 2000; 8: 49–61
Hanefeld M, Fischer S, Schmechel H, et al. Diabetes Intervention Study. Multi-intervention trial in newly diagnosed NIDDM. Diabetes Care 1991; 14: 308–17
Acknowledgements
Dr Maetzel and Dr Wolf had a consultancy relationship with Roche Pharmaceuticals Inc. during execution of this project. Dr Ruof and Ms Covington are employees of Roche Pharmaceuticals Inc. This research was supported by a grant from Roche Pharmaceuticals, Switzerland.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maetzel, A., Ruof, J., Covington, M. et al. Economic Evaluation of Orlistat in Overweight and Obese Patients with Type 2 Diabetes Mellitus. Pharmacoeconomics 21, 501–512 (2003). https://doi.org/10.2165/00019053-200321070-00005
Published:
Issue Date:
DOI: https://doi.org/10.2165/00019053-200321070-00005