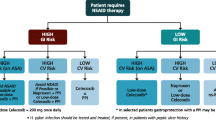
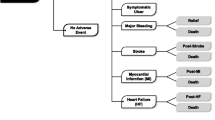
Abstract
Objective:To construct a decision analytical model to compare the costs and clinical consequences of treating patients with celecoxib or various nonsteroidal anti-inflammatory drug (NSAID)/gastrointestinal (GI) co-therapy regimens for the management of osteoarthritis and rheumatoid arthritis. The model quantified the number of patients expected to experience any GI complication commonly associated with NSAID therapy.
Design: Resource use for the treatment of each GI complication in the model was estimated after consulting Canadian experts. Standard unit costs from Ontario were applied to resources to calculate the cost of each complication.
Main outcome measures and results: The model revealed that the NSAID-alone regimen was associated with the lowest cost [$262 Canadian dollars ($Can) per patient per 6 months] followed by the celecoxib regimen ($Can273), diclofenac/misoprostol ($Can365), NSAID + histamine H2 receptor antagonist ($Can413), NSAID + misoprostol ($Can421), and NSAID + proton pump inhibitor ($Can731). A break-even analysis showed that up to 80% of the study cohort could be treated with celecoxib instead of the NSAID-alone regimen without increasing the health system’s overall budget. Celecoxib was associated with the fewest GI-related deaths, hospitalised events, symptomatic ulcers, and cases of anaemia. The celecoxib regimen was also associated with the fewest cases of upper GI distress. Sensitivity analyses revealed that the model was most sensitive to the distribution of GI risk in the population and to the ingredient costs of the treatment alternatives.
Conclusions: This model indicates that the use of celecoxib could lead to the avoidance of a significant number of NSAID-attributable GI adverse events, and the incremental cost of using celecoxib for arthritis patients ≥65 years of age in place of current treatment alternativeswould not impose an excessive incremental impact on a Canadian provincial healthcare budget.






Similar content being viewed by others
References
Mikkelson WM, Duff IF, Dodge HJ. Age-sex specific prevalence of radiographic abnormalities of the joints of the hands, wrists, and cervical spine of adult residents of the Tecumseh, Michigan, Community Health Study area, 1962–1965. J Chronic Dis 1970; 23: 151–9
Bergstrom G, Bjelle A, Sorensen LB, et al. Prevalence of rheumatoid arthritis, osteoarthritis, chondrocalcinosis and gouty arthritis at age 79. J Rheumatol 1986; 13: 527–34
Hawker G. Epidemiology of arthritis and osteoporosis. In: Williams JI, Bradley EM, editors. Patterns of health care in Ontario: arthritis and related conditions. Toronto, Ontario: Institute for Clinical Evaluative Sciences, 1998: 1–10
Coyte P, Asche C, Croxford R, et al. The economic costs of arthritis and rheumatism in Canada. In: Williams JI, Bradley EM, editors. Patterns of health care in Ontario: arthritis and related conditions. Toronto, Ontario: Institute for Clinical Evaluative Sciences, 1998: 27–34
Koch M, Dezi A, Ferrario F, et al. Prevention of nonsteroidal anti-inflammatory drug-induced gastrointestinal mucosal injury. Arch Intern Med 1996; 156 (20): 2321–32
Agrawal NM, Van-Kerckhove HE, Erhardt LJ, et al. Misoprostol coadministeredwith diclofenac for prevention of gastroduodenal ulcers. A one-year study. Dig Dis Sci 1995; 40: 1125–31
Goldstein JL, Larson LR, Yamashita BD, et al. Management of NSAID-induced gastropathy: an economic decision analysis. Clin Ther 1997; 19 (6): 1496–509
Koch M, Capurso L, Dezi A, et al. Prevention of NSAID-induced gastroduodenal mucosal injury: meta-analysis of clinical trials with misoprostol and H2-receptor antagonists. Dig Dis 1995; 13 Suppl. 1: 62–74
Ehsanullah RSB, Page MC, Tildesley G, et al. Prevention of gastroduodenal damage induced by nonsteroidal anti-inflammatory drugs: controlled trial of ranitidine. BMJ 1988; 297: 1017–21
Hudson N, Taha AS, Russell RI. Famotidine for healing and maintenance in nonsteroidal anti-inflammatory drug-associated gastroduodenal ulceration. Gastroenterology 1997; 112 (6): 1817–22
Levine L, Cloud M, Enas N. Nizatidine prevents peptic ulceration in high-risk patients taking nonsteroidal anti-inflammatory drugs. Arch Intern Med 1993; 153: 2449–54
Rugstad HE, Giercksky KE, Husby G, et al. Effect of cimetidine on gastrointestinal symptoms in patients taking nonsteroidal anti-inflammatory drugs. A large double-blind placebo controlled study. Scand J Rheumatol 1994; 23 (4): 177–82
Taha AS, Hudson N, Hawkey CJ, et al. Famotidine for the prevention of gastric and duodenal ulcers caused by nonsteroidal antiinflammatory drugs. N Engl J Med 1996; 334 (22): 1435–9
Hawkey CJ, Karrasch JA, Szczepanski L, et al. Omeprazole compared with misoprostol for ulcers associated with nonsteroidal antiinflammatory drugs. Omeprazole versus Misoprostol for NSAID-induced Ulcer Management (OMNIUM) Study Group. N Engl J Med 1998; 338 (11): 727–34
Bocanegra TS, Weaver AL, Tindall EA, et al. Diclofenac/misoprostol compared with diclofenac in the treatment of osteoarthritis of the knee or the hip: a randomised, placebo controlled trial. Arthrotec Osteoarthritis Study Group. J Rheumatol 1998; 25: 1602–11
Bolten W, Gomes JA, Stead H, et al. The gastroduodenal safety and efficacy of the fixed combination of diclofenac and misoprostol in the treatment of osteoarthritis. Br J Rheumatol 1992; 31 (11): 753–8
Elliott SL, Yeomans ND, Buchanan RRC, et al. Efficacy of 12 months’ misoprostol as prophylaxis against NSAID-induced gastric ulcers. A placebo controlled trial. Scand J Rheumatol 1994; 23 (4): 171–6
Graham DY, White RH, Moreland LW, et al. Duodenal and gastric ulcer prevention with misoprostol in arthritis patients taking NSAIDs. Ann Intern Med 1993; 119 (4): 257–62
Melo-Gomes JA, Roth SH, Zeeh J, et al. Double-blind comparison of efficacy and gastroduodenal safety of diclofenac/misoprostol, piroxicam, and naproxen in the treatment of osteoarthritis. Ann Rheum Dis 1993; 52 (12): 881–5
Raskin JB, White RH, Jackson JE, et al. Misoprostol dosage in the prevention of nonsteroidal anti-inflammatory drug-induced gastric and duodenal ulcers: a comparison of three regimens. Ann Intern Med 1995; 123 (5): 344–50
Roth SH. Misoprostol in the prevention of NSAID-induced gastric ulcer: a multicenter, double-blind, placebo-controlled trial. J Rheumatol Suppl. 1990 Feb; 20: 20–4
Silverstein FE, Graham DY, Senior JR, et al. Misoprostol reduces serious gastrointestinal complications in patients with rheumatoid arthritis receiving nonsteroidal anti-inflammatory drugs. A randomized, double-blind, placebo-controlled trial. Ann Intern Med 1995; 123: 241–9
Verdickt W, Moran C, Hantzschel H, et al. Adouble-blind comparison of the gastroduodenal safety and efficacy of diclofenac and a fixed dose combination of diclofenac and misoprostol in the treatment of rheumatoid arthritis. Scand J Rheumatol 1992; 21 (2): 85–91
Goldstein JL, Larson LR, Yamashita BD. Prevention of nonsteroidal anti-inflammatory drug-induced gastropathy: clinical and economic implications of a single-tablet formulation of diclofenac/misoprostol. Am J Manag Care 1998; 4: 687–97
Simon LS, Weaver AL, Graham DY, et al. The anti-inflammatory and upper gastrointestinal effects of celecoxib in rheumatoid arthritis: a randomized controlled trial. JAMA 1999; 282: 1921–8
Bensen WG, Fiechtner JJ, McMillen JI, et al. Treatment of osteoarthritis with celecoxib, a cyclooxygenase-2 inhibitor: a randomized controlled trial. Mayo Clin Proc 1999; 74 (11): 1095–105
Fries JF. NSAID gastropathy: the second most deadly rheumatic disease? Epidemiology and risk appraisal. J Rheumatol Suppl. 1991 Mar; 28: 6–10
Burke TA, Zabinski RA, Pettitt D, et al. A framework for evaluating the clinical consequences of initial therapy with NSAIDs, NSAIDs plus gastroprotective agents, or celecoxib in the treatment of arthritis. Pharmacoeconomics 2001; 19 Suppl. 1: 33–47
Fries JF, Williams CA, Bloch DA, et al. Nonsteroidal anti-inflammatory drug-associated gastropathy: incidence and risk factor models. Am J Med 1991; 91: 213–22
Bensen WG, Agrawal NM, Zhao SZ, et al. Upper gastrointestinal (UGI) tolerability of celecoxib, a COX-2 specific inhibitor, compared to naproxen and placebo. Arthritis Rheum 1999; 42 (9 Suppl.): S142
Chevat C, Peña BMet al, AlMJ. Healthcare resource utilisation and costs of treating NSAID-associated gastrointestinal toxicity: a multinational perspective. Pharmacoeconomics 2001; 19 Suppl. 1: 17–32
Talley J, Evans J, Fleming K, et al. Nonsteroidal antiinflammatory drugs and dyspepsia in the elderly. Dig Dis Sci 1995; 40 (6): 1345–50
Kephart G, Sketris I, Smith M, et al. Coprescribing of nonsteroidal anti-inflammatory drugs and cytoprotective and antiulcer drugs in Nova Scotia’s senior population. Clin Ther 1995; 17 (6): 1159–73
Hogan DB, Campbell NR, Crutcher R, et al. Prescription of nonsteroidal anti-inflammatory drugs for elderly people in Alberta. Can Med Assoc J 1994; 151: 315–22
Rahme E, Joseph L, Kong SX, et al. Cost of gastro-protective agents attributable to nonsteroidal antiinflammatory drugs [abstract no. 063]. 14th International Conference on Pharmacoepidemiology; 1998 Aug 16–19; Berlin, Germany; Pharmacoepidemiology and Drug Safety 1999; 8 Suppl. 2: S102
Scholes D, Stergachis A, Penna PM, et al. Nonsteroidal antiinflammatory drug discontinuation in patients with osteoarthritis. J Rheumatol 1995; 22 (4): 708–12
Smalley WE, Griffin ME. The risks and costs of upper gastrointestinal disease attributable to NSAIDs. Gastroenterol Clin North Am 1996; 25 (2): 373–96
Morgan GJ, Poland M, DeLapp RE. Efficacy and safety of nabumetone versus diclofenac, naproxen, ibuprofen and piroxicam in the elderly. Am J Med 1993; 95 Suppl. 2A: 19s-27
Acknowledgements
The authors gratefully acknowledge the assistance of Dan Pettitt, Kathleen Villa and Kurt Henke. This study was supported by Pharmacia Corporation and Pfizer, Inc.
Author information
Authors and Affiliations
Corresponding author
Additional information
At the time this research was conducted, Dr Zabinski was an employee of Pharmacia Corporation. His current affiliation is Express Scripts Inc., Bloomington, Minnesota, USA.
Rights and permissions
About this article
Cite this article
Zabinski, R.A., Burke, T.A., Johnson, J. et al. An Economic Model for Determining the Costs and Consequences of Using Various Treatment Alternatives for the Management of Arthritis in Canada. Pharmacoeconomics 19 (Suppl 1), 49–58 (2001). https://doi.org/10.2165/00019053-200119001-00004
Published:
Issue Date:
DOI: https://doi.org/10.2165/00019053-200119001-00004