Abstract
Objective: To estimate the short term and long term cost effectiveness, from a healthcare perspective, associatedwith the introduction of lamivudine for chronic hepatitis B.
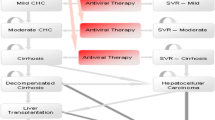
Design:The analysis used a 2-step modelling approach. A decision tree was used to estimate clinical outcomes and costs after 1 year. The 1-year results were then extrapolated to 70 years using a Markov model.
Patients: The study population comprised hypothetical cohorts of patients with chronic hepatitis B, representative of those likely to receive treatment in clinical practice in Australia.
Main outcome measures and results: In the short term, more patients seroconvertedwhen lamivudinewas available, with an incremental cost-effectiveness ratio of 3341 Australian dollars ($A) per additional seroconversion. In the long term, the introduction of lamivudine increased life expectancy by 3.9 years [3.2 quality-adjusted life-years (QALYs)] compared with when interferon-α was the only treatment, or 4.6 years (3.8 QALYs) comparedwith no treatment. There were reductions in lifetime risk of developing compensated cirrhosis, decompensated cirrhosis and hepatocellular carcinoma of 5, 11 and 11%, respectively, when lamivudine was available. The incremental cost of having lamivudine available, as opposed to interferon-α only, was $A633 per year of life saved or $A735 per QALY.
Conclusion: The introduction of lamivudine is expected to reduce and delay the progression of chronic hepatitis B, increasing the life expectancy and quality of life of patients for a small overall increase in healthcare costs.
















Similar content being viewed by others
References
World Health Organization. Hepatitis B fact sheet WHO/204 [internet web page]. Available from: URL: http://www.who.int/inf-fs/en/fact204.html [Accessed 2000 Mar 13]
Purcell RH. The discovery of the hepatitis viruses. Gastroenterology 1993; 104: 955–63
Davey S. State of the world’s vaccines and immunisation. Geneva: World Health Organization, 1996
Farell GC. Chronic viral hepatitis. Med J Aust 1998; 168: 619–26
Mast EE, Alter MJ. Epidemiology of viral hepatitis: an overview. Semin Virol 1993; 4: 273–83
Lee WM. Hepatitis B virus infection. N Engl J Med 1997; 337: 1733–44
Gust ID. Immunisation against hepatitis B in Taiwan. Gut 1996; 38 Suppl. 2: S67–8
Nicoll A, Locarnini S. Present and future directions in the treatment of chronic hepatitis B infection. J Gastroenterol Hepatol 1997; 12: 843–53
Niederau C, Heintges T, Lange S, et al. Long term follow up of HBeAg-positive patients treated with interferon alfa for chronic hepatitis B. N Engl J Med 1996; 334: 1422–7
Lau DT, Everhart J, Kleiner DL, et al. Long term follow-up of patients with chronic hepatitis B treated with interferon alpha. Gastroenterology 1997; 113: 1660–7
Hope RL, Welman M, Dingley J, et al. Interferon alpha for chronic active hepatitis B, long term follow-up of 62 patients: outcomes and predictors of response. Med J Aust 1995; 162: 8–11
Tine F, Liberati A, Craxi A, et al. Interferon treatment in patients with chronic hepatitis B; a meta-analysis of the published literature. J Hepatol 1993; 18: 154–62
Lampertico P, Del Ninno E,Maniz A, et al. A randomised, controlled trial of a 24-month course of interferon alfa 2b in patients with chronic hepatitis B who had hepatitis B virus DNA without hepatitis B e antigen in serum. Hepatology 1997; 26: 1621–5
Burt MJ, Ross AG, Schroeder BA, et al. Evaluation of alpha interferon for the treatment of chronic hepatitis B infection in Christchurch. N Z Med J 1996; 109: 162–4
Wong DK, Cheung AM, O’Rourke K, et al. Effect of alpha interferon treatment in patients with hepatitis B e antigen-positive chronic hepatitis B: a meta-analysis. Ann Intern Med 1993; 119: 312–23
Hoofnagle JH. Therapy of viral hepatitis. Digestion 1998; 59: 563–78
Krogsgaard K, Bindslev N, Christensen E, et al. The treatment effect of alpha interferon in chronic hepatitis B is independent of pretreatment variables: results based on individual patient data from 10 clinical controlled trials. J Hepatol 1994; 21: 646–55
Krogsgaard D, The Long-term Follow-up Investigator Group, The European Study Group on Viral Hepatitis (EUROHEP) Executive Team on Antiviral Treatment. The long term effect of treatment with interferon alpha 2A in chronic hepatitis B. J Viral Hepat 1998; 5: 389–97
Lok AS, Chung HT, Liu VW, et al. Long-term follow-up of chronic hepatitis B patients treated with interferon alfa. Gastroenterology 1993; 105: 1833–8
Lok AS, Lai CL, Wu PC, et al. Long-term follow-up in a randomised controlled trial of recombinant alpha2-interferon in Chinese patients with chronic hepatitis B infection. Lancet 1988; II: 298–302
Guptan RK, Thakur V, Malhortra V, et al. Low-dose recombinant interferon therapy in anti HBe-positive chronic hepatitis B in Asian Indians. J Gastroenterol Hepatol 1998; 13: 675–9
Zhang X, Zoulim F, Haberseber I, et al. Analysis of hepatitis B virus genotypes and pre-core region variability during interferon treatment of Hbe antigen negative chronic hepatitis B. J Med Virol 1996; 48: 8–16
Dejongh FE, Janssen HL, DeMan RA, et al. Survival and prognostic indicators in hepatitis B surface antigen-positive cirrhosis of the liver. Gastroenterology 1992; 103: 1630–5
Lai CL, Chien RN, Leung NW, et al. A one-year trial of lamivudine for chronic hepatitis B. N Engl J Med 1998; 339: 61–8
Dienstag J, Schiff E, Wright T, et al. Lamivudine treatment for one year in previously untreated US hepatitis B patients: histological improvement and hepatitis e-antigen (HBeAg) seroconversion [abstract]. Gastroenterology 1998; 114: A1235
Schiff E, Karayalcin S, Grimm I, et al. A placebo controlled study of lamivudine and interferon alpha-2b in patients with chronic hepatitis B who had previously failed interferon therapy [abstract]. Hepatology 1998; 28 (4 Pt 2): 388A
Heathcote J, Schalm SW, Cianciara J, et al. Lamivudine and Intron A combination treatment in patients with chronic hepatitis B infection [abstract]. J Hepatol 1998; 28 Suppl. 1: 43
Nevens F, Main J, Honkoop P, et al. Lamivudine therapy for chronic hepatitis B: a six month randomised dose-ranging study. Gastroenterology 1997; 113: 1258–63
Dienstag JL, Perrillo RP, Schiff ER, et al. A preliminary trial of lamivudine for chronic hepatitis B infection. N Engl J Med 1995; 333: 1657–61
Goodman Z, Dhillon AP, Wu PC, et al. Lamivudine treatment reduces progression to cirrhosis in patients with chronic hepatitis B [abstract]. J Hepatol 1999; 30 Suppl. 1: 59
Lueng N, Wu PC, Tsang S, et al. Continued histological improvement in Chinese patients with chronic hepatitis B with 2 years lamivudine [abstract]. Hepatology 1998; 28: 489A
Liaw YF, Lai CL, Leung NWY, et al. Two-year lamivudine therapy in chronic hepatitis B infection: results of a placebo controlled multi-centre study in Asia [abstract]. Gastroenterology 1998; 114: 1289A
Lueng NWY, Lai CL, Chang TT, et al. Three year lamivudine therapy in chronic HBV [abstract]. J Hepatol 1999; 30 Suppl. 1: 59
Van Thiel DH, Friedlander L, Kania R, et al. Lamivudine treatment of advanced and decompensated liver disease due to hepatitis B. Hepatogastroenterology 1997; 44: 808–12
Picardi M, Selleri C, De Rosa G, et al. Lamivudine treatment for chronic replicative hepatitis B virus infection after allogenic bone marrow transplantation. Bone Marrow Transplant 1998; 21: 1267–9
Chan TM, Wu PC, Li FK, et al. Treatment of fibrosing cholestatic hepatitis with lamivudine. Gastroenterology 1998; 115: 177–81
Faraidy KA, Yoshida EM, Davis JE, et al. Alteration of the dismal natural history of fibrosing cholestatic hepatitis secondary to hepatitis B virus with the use of lamivudine. Transplantation 1997; 64: 926–8
Kaldor JM, Plant AJ, Thompson SC, et al. The incidence of hepatitis B infection in Australia: an epidemiological review. Med J Aust 1996; 165: 322–6
Wong JB, Koff RS, Tine F, et al. Cost-effectiveness of interferon-alpha 2B treatment for hepatitis B e antigen-positive chronic hepatitis B. Ann Intern Med 1995; 122: 664–75
Wong JB. Interferon treatment for chronic hepatitis B or C infection: costs and effectiveness. Acta Gastroenterol Belg 1998; 61: 238–42
Dushieko GM, Roberts JA. Treatment of chronic type B and C hepatitis with interferon alpha: an economic appraisal. Hepatology 1995; 22: 1863–73
Commonwealth Department of Health and Aged Care. Schedule of pharmaceutical benefits. Silverwater: McMillan Printing Group, 1999
Commonwealth Department of Health and Aged Care. Medicare benefits schedule book. Canberra: Australian Government Publishing Service, 1999
Department of Human Services, Victoria. Victorian Acute Health Cost Weight Study, 1996–7, prepared by Health Solutions International. Melbourne: Department of Human Services, 1997
Korenman J, Baker B, Waggoner J, et al. Long-term remission of chronic hepatitis B after alpha-interferon therapy. Ann Intern Med 1991; 114: 629–34
Carreflo V, Bartolome J, Calstillo I. Long-term effect of interferon therapy in chronic hepatitis B. J Hepatol 1994; 20: 431–5
Schiff E, Cianciuru Y, Kowdley K, et al. Durability of HBeAg seroconversion after lamivudine monotherapy in controlled phase II and III trials [abstract]. Hepatology 1998; 28: 163A
Liaw YF, Tai DJ, Chu CM, et al. The development of cirrhosis in patients with chronic type B hepatitis: a prospective study. Hepatology 1988; 5: 493–6
Fattovich G, Brollo L, Giustina G, et al. Natural history and prognostic factors for chronic hepatitis type B. Gut 1991; 32: 294–8
Australian Bureau of Statistics. Life tables. Canberra: Australian Bureau of Statistics, 1997. (Data on disk)
Beasley RP, Hwang LY, Lin CC, et al. Hepatocellular carcinoma and hepatitis B virus. Lancet 1981; II: 1129–33
Lo K-J, Toy MJ, Chien M-C, et al. The natural course of hepatitis B surface antigen-positive chronic active hepatitis in Taiwan. J Infect Dis 1982; 146: 205–10
Liaw Y-F, Lin D-Y, Chen T-J, et al. Natural course after the development of cirrhosis in chronic type B hepatitis: a prospective study. Liver 1989; 9: 235–41
McMahon BJ, Alberts SR, Wainwright RB, et al. Hepatitis B related sequelae: prospective study in 1400 hepatitis B surface antigen-positive Alaskan native carriers. Arch Intern Med 1990; 150: 1051–4
Fattovich G, Giustina G, Schalm SW, et al. Occurrence of hepatocellular carcinoma and decompensation in Western European patients with cirrhosis type B: the EUROHEP Study Group in hepatitis B virus and cirrhosis. Hepatology 1995; 21: 77–82
International Interferon-alpha Hepatocellular Carcinoma Study Group. Effect of interferon-alpha on progression to cirrhosis to hepatocellular carcinoma: a retrospective cohort study. Lancet 1998; 351: 1535–9
Columbo M, De Franchis R, Del Ninno E, et al. Hepatocellular carcinoma in patients with cirrhosis. N Engl JMed 1991; 325: 675–80
Realdi G, Fattovich G, Hadziyannis S, et al. Survival and prognostic factors in 366 patients with compensated cirrhosis type B: a multicenter study. J Hepatol 1994; 21: 656–66
Department of Human Services South Australian Cancer Registry Data. Epidemiology of cancer in South Australia 1977–96. Data on file
Hawthorne G, Richardson J, Osborne R, et al. The Assessment of Quality of Life (AQOL) instrument: construction, initial validation and utility scaling. Working paper 76. Melbourne: Centre for Health Program Evaluation, 1997
Hawthorne G, Richardson J, Osborne R, et al. The Assessment of Quality of Life (AQOL) instrument: a psychometric measurement of health related quality of life. Qual Life Res 1999; 8: 209–24
Bennett WG, Inoie Y, Beck JR, et al. Estimates of the cost-effectiveness of a single course of interferon-alpha 2b in patients with histologically mild chronic hepatitis C. Ann Intern Med 1997; 127: 855–65
Data on file, Commonwealth Department of Health and Aged Services, National Hospital Cost Data Collection Public Sector 1996/7, 1998
Johannesson M. The impact of age on the cost-effectiveness of hypertension treatment: an analysis of randomised drug trials. Med Decis Making 1994; 14: 236–44
Robinson R. Cost-utility analysis. BMJ 1993; 307: 859–62
Sarin SK. Hepatitis B mutants: prevalence, significance and therapy [editorial]. Hepatit World 1998; 3: 1
Tassopoulos NC, Volpes R, Pastore G, et al. Efficacy of lamivudine in patients with hepatitis B e antigen-negative/ hepatitis B virus DNA-positive (precoremutant) chronic hepatitis B. Hepatology 1999; 29: 889–96
Chayama K, Suzuki Y, Kobaysashi M, et al. Emergence and takeover of YMDD motif mutant hepatitis B virus during long-term lamivudine therapy and re-takeover by wild type after cessation of therapy. Hepatology 1998; 27: 1711–6
Honkoop P, de Man RA, Niesters H, et al. Clinical impact of lamivudine resistance in chronic hepatitis B. J Hepatol 1998; 29: 510–4
Perillo RP, Schalm SW, Schiff ER, et al. Predictors of HBeAg seroconversion in chronic hepatitis B patients treated with lamivudine [abstract]. Hepatology 1999; 30 (4 Pt 2): 317A
Acknowledgements
This study was funded by GlaxoWellcome Australia Limited. We would like to thank the members of the Australian Hepatitis B Expert Panel for their input: Dr Paul Desmond, Professor Graeme Cooksley (Royal Brisbane Hospital, QLD), Dr Darrell Crawford (Princess Alexandra Hospital, QLD), Professor Geoffrey Farrell (Westmead Hospital, NSW), Associate Professor GW McCaughan (Royal Prince Alfred Hospital, NSW) and Dr William Sievert (Monash Medical Centre, VIC).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Crowley, S., Tognarini, D., Desmond, P.V. et al. Cost-Effectiveness Analysis of Lamivudine for the Treatment of Chronic Hepatitis B. Pharmacoeconomics 17, 409–427 (2000). https://doi.org/10.2165/00019053-200017050-00001
Published:
Issue Date:
DOI: https://doi.org/10.2165/00019053-200017050-00001