Abstract
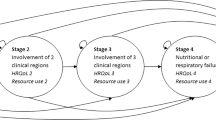
Objective: Amyotrophic lateral sclerosis (ALS) is a fatal, degenerative neuromuscular disease characterised by a progressive loss of voluntary motor activity. Recombinant human insulin-like growth factor I (rhIGF-I) has been shown to be useful in treating ALS. The purpose of this study was to examine the cost effectiveness of rhIGF-I therapy in patients who have ALS.
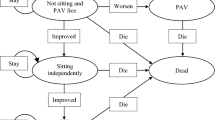
Design: We performed a cost-effectiveness analysis from the societal perspective on 177 patients who received treatment with rhIGF-I or placebo in a North American randomised clinical trial. We estimated the incremental cost-effectiveness ratio of rhIGF-I using resource utilisation and functional status measurements from the clinical trial. Costs were estimated from 1996 US Medicare reimbursement schedules. Utility weights were elicited from ALS healthcare providers using the standard gamble technique.
Main outcome measures and results: The overall cost per quality-adjusted lifeyear (QALY) gained for rhIGF-I therapy compared with placebo was $US67 440. For the subgroups of patients who were progressing rapidly or were in earlier stages of disease at enrolment, rhIGF-I cost $US52 823 and $US43 197 per QALY gained, respectively.
Conclusions: Treatment with rhIGF-I is most cost effective in ALS patients who are either in earlier stages of the disease or progressing rapidly. The cost effectiveness of rhIGF-I therapy compares favourably with treatments for other chronic progressive diseases.
Similar content being viewed by others
References
Incidence & Prevalence (Database Online). Mountain view: timely data resources. 1997; Nov 1996
Klein LM, Forshew DA. The economic impact of ALS. Neurology 1996; 47 Suppl. 2: 126S–9S
Lewis ME, Neff NT, Contreras PC, et al. Insulin-like growth factor-I for treatment of motor neuronal disorders. Exp Neurol 1993; 124: 73–88
D’Ercole AJ, Ye P, Calikoglu AS, et al. The role of insulin-like growth factors in the central nervous system. Mol Neurobiol 1996; 13: 227–55
The Pink Sheet. FDC Reports 1996; June 24: T&G 2–3
Lai EC, Felice KJ, Festoff BW, et al. Effect of recombinant human insulin-like growth factor I on progression of ALS: a placebo-controlled study. Neurology 1997; 49: 1621–30
Oddone EZ, Cowper P, Hamilton JD, et al. Cost effectiveness analysis of early zidovudine treatment of HIV infected patients. BMJ 1993; 307: 1322–5
Garner TI, Dardis R. Cost-effectiveness analysis of end-stage renal disease treatments. Med Care 1987; 25–34
Kattan MW, Inoue Y, Giles FJ, et al. Cost-effectiveness of interferon-alpha and conventional chemotherapy in chronic myelogenous leukemia. Ann Intern Med 1996; 125: 541–8
Haverkamp LJ, Appel V, Appel SH. Natural history of amyotrophic lateral sclerosis in a database population. Validation of a scoring system and a model for survival prediction. Brain 1995; 118: 707–9
Appel V, Stewart SS, Smith G, et al. A rating scale for amyotrophic lateral sclerosis: description and preliminary experience. Ann Neurol 1987; 22: 328–33
Bergner M, Bobbitt RA, Carter WB, et al. The Sickness Impact Profile: development and final revision of a health status measure. Med Care 1981; 19: 787–805
McGuire D, Garrison L, Armon C, et al. Relationship of the Tufts Quantitative Neuromuscular Exam (TQNE) and the Sickness Impact Profile (SIP) in measuring progression of ALS. SSNJV/CNTF ALS Study Group. Neurology 1996; 46: 1442–4
Schulman KA, Rubenstein LE, Glick HA, et al. Relationships between sponsors and investigators in pharmacoeconomic and clinical research. Pharmacoeconomics 1995; 7: 206–20
Hillman AL, Eisenberg JM, Pauly MV, et al. Avoiding bias in the conduct and reporting of cost-effectiveness research sponsored by pharmaceutical companies. N Engl J Med 1991; 324: 1362–5
Lipscomb J, Weinstein MC, Torrance GW. Time preference. In: Gold MR, Weinstein MC, editors. Cost-effectiveness in health and medicine. New York: Oxford University Press, 1996: 214–35
Norris FH. Care of the amyotrophic lateral sclerosis patient. In: H Mitsumoto, FH Norris, editors. Amyotrophic lateral sclerosis. A comprehensive guide to management. New York: Demos Publications, 1994: 39
O’Brien BJ, Drummond MF, Labelle RJ, et al. In search of power and significance: Issues in the design and analysis of stochastic cost-effectiveness studies in health care. Med Care 1994; 32: 150–63
Sacristan JA, Day SJ, Navarro O, et al. JM. Use of confidence intervals and sample size calculations in health economics studies. Ann Pharmacother 1995; 29: 719–25
Gardiner J, Hogan A, Holmes-Rovner M, et al. Confidence intervals for cost-effectiveness ratios. Med Decis Making 1995; 15: 254–63
Fenn P, McGuire A, Phillips V, et al. The analysis of censored treatment cost data in economic evaluation. Med Care 1995; 33: 851–63
Ganiats TG, Miller CJ, Kaplan RM. Comparing the quality-adjusted life-year output of two treatment arms in a randomized trial. Med Care 1995; 33: 245AS–54AS
Lee ET. Analytical estimation procedures for survival distributions. In: Statistical methods for survival data analysis. Wiley series in probability and mathematical statistics. New York: John Wiley and Sons, Inc; 1992
Glasziou PP, Simes RJ, Gelber RD. Quality adjusted survival analysis. Stat Med 1990; 9: 1259–76
Sonnenberg FA, Beck JR. Markov models in medical decision making: a practical guide. Med Decis Making 1993; 13: 322–38
Drummond MF. Discussion: Torrance’s ‘Utility approach to measuring health-related quality of life.’ J Chron Dis 1987; 40: 601–3
Patrick DL, Starks HE, Cain KC, et al. Measuring preferences for health states worse than death. Med Decis Making 1994; 14: 9–18
Torrance GW. Designing and conducting cost-utility analyses. In: Spilker B, editor. Quality of life and pharmacoeconomics in clinical trials. 2nd ed. Philadelphia: Lippincott-Raven Publishers, 1996
Health Care Financing Administration. 1996 Medicare program: Revisions to payment policies and adjustments to the relative value units under the physician fee schedule for calendar year 1996. Federal Register 1996
Samet JH, Libman H, Steger KA, et al. Compliance with zidovudine therapy in patients infected with human immunodeficiency virus, type 1: a cross-sectional study in a municipal hospital clinic. Am J Med 1992; 92: 495–502
Briggs A, Sculpher M, Buxton M. Uncertainty in the economic evaluation of health care technologies: the role of sensitivity analysis. Health Econ 1994; 3: 95–104
Mooney CZ, Duval RD. Bootstrapping: a nonparametric approach to statistical inference. Newbury Park; Sage Publications Inc. 1993
Coyle D. Statistical analysis in pharmacoeconomic studies. Pharmacoeconomics 1996; 9: 506–16
Ringle SP, Murphy JR, Alderson MK, et al. The natural history of amyotrophic lateral sclerosis. Neurology 1993; 43: 1316–22
Munsat TL, Andres PL, Finison L, et al. The natural history of motoneuron loss in amyotrophic lateral sclerosis. Neurology 1988; 38: 409–13
Gubbay SS, Kahana E, Zilber N, et al. Amyotrophic lateral sclerosis. A study of its presentation and prognosis. Neurology 1985; 232: 295–300
Norris F, Shepherd R, Denys E UK, et al. Onset, natural history and outcome in idiopathic adult motor neuron disease. J Neurol Sci 1993; 118: 48–55
Caroscio JT, Mulvihill MN, Sterling R, et al. Amyotrophic lateral sclerosis. It’s natural history. Neurol Clin 1987; 5: 1–8
Rosen AD. Amyotrophic lateral sclerosis. Clinical features and prognosis. Arch Neurol 1978; 35: 638–42
Welch HG, Larson EB. Cost-effectiveness of bone marrow transplantation in acute nonlymphocytic leukemia. N Engl J Med 1989; 321: 807–12
Evans RW. Cost-effectiveness analysis of transplantation. Surg Clin North Am 1986; 66: 603–16
US Bureau of the Census. Statistical abstract of the United States 1997. 117th ed. Washington, DC: US Bureau of the Census, 1997
Torrance GW. Utility approach to measuring health-related quality of life. J Chron Dis 1987; 40: 593–600
Laupacis A, Feeny D, Detsky AS, et al. How attractive does a new technology have to be to warrant adoption and utilization? Tentative guidelines for using clinical and economic evaluations. Can Med Assoc J 1992; 146: 473–81
Gold MR, Patrick DL, Torrance GW, et al. Identifying and valuing outcomes. In: Gold MR, Siegel JE, Russell LB, et al., editors. Cost-effectiveness in health and medicine. New York: Oxford University Press, 1996: 82–123
McDonald ER. Psychosocial-spiritual overview. In: H Mitsumoto, FH Norris, editors. Amyotrophic lateral sclerosis: a comprehensive guide to management. New York: Demos Publications, 1994: 206–22
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ackerman, S.J., Sullivan, E.M., Beusterien, K.M. et al. Cost Effectiveness of Recombinant Human Insulin-Like Growth Factor I Therapy in Patients with ALS. Pharmacoeconomics 15, 179–195 (1999). https://doi.org/10.2165/00019053-199915020-00006
Published:
Issue Date:
DOI: https://doi.org/10.2165/00019053-199915020-00006