Summary
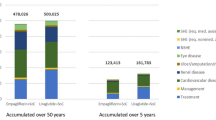
Bodyweight is an acknowledged independent risk factor for coronary heart disease (CHD). The present model analysis was undertaken to investigate the clinical and economic impact of bodyweight gain in patients with type 2 (non— insulin—dependent) diabetes mellitus and its effects on the development of CHD. Based on a retrospective re—evaluation of data from the Diabetes Intervention Study (DIS), patients with type 2 diabetes mellitus and stable body—weight (group A) had a significantly lower rate of combined CHD events (30.3%) than patients showing a bodyweight gain (group B; 38.2%) over 10 years. Prevention of bodyweight gain, therefore, appears to be a meaningful strategy in the management of diabetes mellitus. In addition to this clinical advantage, prevention of CHD will also result in economic savings associated with avoided treatment of coronary events. Based on the clinical outcomes from the DIS, the calculated per—patient net savings for a patient with type 2 diabetes mellitus and stable bodyweight amounted to 1085 deutschmarks (DM) when compared with a patient experiencing a bodyweight increase.
In a further step, the above situation was projected to current type 2 diabetes mellitus practice. Oral first—line treatment of type 2 diabetes mellitus is usually initiated with glibenclamide (glyburide), which is known to increase bodyweight (reflecting group B). The novel α—glucosidase inhibitor acarbose, in contrast, appears to be as effective as glibenclamide, but has the advantage of being body—weight— neutral (reflecting group A). From the clinical viewpoint, acarbose can thus be considered an alternative to glibenclamide. From the viewpoint of drug costs, monotherapy with acarbose is 4 times as expensive as glibenclamide in Germany, resulting in per-patient incremental costs of DM3527 for acarbose over 10 years. Balanced against the potential 10−year cost saving of DM1085 resulting from the potential of acarbose to prevent CHD, around one—third of the incremental cost of acarbose may be recouped by this single effect. However, further possible benefits of acarbose, including the avoidance of hypoglycaemia and the deferral of costly insulin therapy, may improve the economic value of this novel antidiabetic agent. Given the indirect approach of this evaluation and its many limitations, the above findings need critical appraisal, and comparative trials are urgently required to substantiate our preliminary results.
Similar content being viewed by others
References
Dornan T. Diabetes in the elderly: epidemiology. J R Soc Med 1994; 87: 609–12
Garcia MJ, Mc Namara PM, Gordon T, et al. Morbidity and mortality in diabetics in the Framingham population: sixteen year follow—up study. Diabetes 1974; 23: 105–11
Morrish NJ, Stevens LK, Head J, et al. A prospective study of mortality among middle—aged diabetic patients (the London Cohort of the WHO Multinational Study of Vascular Disease in Diabetics) II: associated risk factors. Diabetologia 1990; 33: 542–8
Songer TJ. The economic costs of NIDDM. Diabetes Metab Rev 1992; 8 (4): 389–404
Dinkel R, Banz K. Management of diabetes: socioeconomic aspects. Medicographia 1995; 17 (2): 35–8
Huse DM, Oster G, Killen AR, et al. The economic costs of non—insulin—dependent diabetes mellitus. JAMA 1989; 19: 2708–13
Fölsch UR, Lembcke B. Inhibition of intestinal alphaglucosidases in the treatment of diabetes mellitus. Internist 1991; 32: 699–707
Hanefeld M, Fischer S, Schulze J, et al. Therapeutic potentials of acarbose as first—line drug in NIDDM insufficiently treated with diet alone. Diabetes Care 1991; 14: 732–7
Hoffmann J. Acarbose und glibenclamid bei typ—II—diabetes. Münch Med Wochenschr 1990; 31/32: 487–90
Spengler M, Hänsel G, Boehme K. Acarbose und glibenclamid bei typ—II—diabetes. Z Allgemeinmed 1990; 66: 606–10
Chiasson J-L, Josse RG, Hunt JA, et al. The efficacy of acarbose in the treatment of patients with non—insulin—dependent diabetes mellitus. Ann Intern Med 1994; 121: 928–35
Alberti KGMM. NIDDM: the forgotten millions. IDF Bull 1995; 40 (2): 8–9
Donahue RP, Abbott RD, Bloom E, et al. Central obesity and coronary heart disease in men. Lancet 1987 April 11; I: 821–4
Hanefeld M, Schmechel H, Julius U, et al. Five—year incidence of coronary heart disease related to major risk factors and metabolic control in newly diagnosed non—insulin—dependent diabetes. Nutr Metab Cardiovasc Dis 1991; 1: 135–40
Manson J, Colditz GA, Stampfer MJ, et al. A prospective study of obesity and risk of coronary heart disease in women. N Engl J Med 1990; 322: 882–92
Obesity. London: Office of Health Economics, 1994
Pekkanen J, Tervahauta M, Nissinen A, et al. Does the predictive value of baseline coronary risk factors change over a 30−year follow—up? Cardiology 1993; 82: 181–90
Ravussin E, Swinburn BA. Pathophysiology of obesity. Lancet 1992; 340: 404–8
Ashley FW, Kannel WB. Relation of weight change to changes in atherogenic traits: the Framingham study. J Chronic Dis 1974; 27: 103–14
Hanefeld M. Principles of treatment in NIDDM. In: Lefèbvre PJ, Standl E, editors. New aspects in diabetes. New York: Walter de Gruyter Berlin, 1992: 150–61
Tattersall RB. Combined insulin and tablet treatment in sulphonylurea failures. Diabetes Nutr Metab 1990; 3 Suppl. 1: 35–45
United Kingdom Prospective Diabetes Study Group. United Kingdom Prospective Diabetes Study (UKPDS) 13: relative efficacy of randomly allocated diet, sulphonylurea, insulin or metformin in patients with newly diagnosed non—insulin dependent diabetes followed for three years. BMJ 1995; 310: 83–8
Wolffenbuttel BH, Weber RF, van Koetsveld PM, et al. A randomised crossover study of sulphonylurea and insulin treatment in patients with type 2 diabetes poorly controlled on dietary therapy. Diabet Med 1989; 6 (6): 520–5
Hanefeld M, Fischer S, Schmechel H, et al. The Diabetes Intervention Study: multi—interventional trial in newly diagnosed type 2 diabetes. Diabetes Care 1991; 14 (4): 308–17
Rose GA, Blackburn H, Gillum RF, et al. Cardivascular survey methods. 2nd ed. Geneva: World Health Organization (WHO), 1982. WHO monograph series no.: 56
Wezel H, Liebhold R, editors. Handkommentar: bewertungsmaβst’ab für kassenärztliche Leistungen (BMÄ’ 87), Ersatzkassen—Gebührenordnung (E-GO’ 87), Amtliche Gebührenordnung für Ärzte (GOÄ). St. Augustin: Asgard Verlag, 1995
Rote Liste 1995. Bundesverband der pharmazeutischen industrie. Frankfurt: Rote Liste GmbH, 1995
Statistisches Jahrbuch für die Bundesrepublik Deutschland 1995. Wiesbaden: Statistisches Bundesamt, 1995
Kübler W, Niebauer J, Kreuzer J, et al. Stationäre und ambulante rehabilitation. Münch Med Wochenschr 1994; 136 (27): 418–23
Jennings AM, Wilson RM, Ward JD. Symptomatic hypoglycemia in NIDDM patients treated with oral hypoglycemic agents. Diabetes Care 1989; 12 (3): 203–8
Seltzer HS. Drug—induced hypoglycemia: a review based on 473 cases. Diabetes 1972; 21: 955–66
Asplund K, Wilholm BE, Lithner F. Glibenclamide—associated hypoglycaemia: a report on 57 cases. Diabetologia 1983; 24: 412–7
Berger W, Caduff F, Pasquel M, et al. Die relative Häufigkeit der schweren Sulfonylharnstoff—Hypoglykämie in den letzten 25 Jahren in der Schweiz. Schweiz Med Wochenschr 1986; 116: 145–51
Federlin F. Das Risiko therpiebedingter Hypoglykämien. In: Waldhäusl W, Gries FA, editors. Diabetes in der praxis. Berlin: Springer Verlag, 1993: 218–32
Campbell IW. Metformin and the sulphonylureas: the comparative risks. Horm Metab Res Suppl 1985; 15: 105–11
Lebovitz HE, Melander A. Sulfonylureas: basic aspects and clinical uses. In: Alberti KGMM, Defronzo RA, Keen H, et al. International textbook of diabetes mellitus. Chichester: John Wiley & Sons, 1992: 746–72
Gerich JE. Oral hypoglycemic agents. N Engl J Med 1989; 321 (18): 1231–45
Yee HS, Fong NT. A review of the safety and efficacy of acrbose in diabetes mellitus. Pharmacotherapy 1996; 16 (5): 792–805
Campbell LK, White JR, Campbell RK. Acarbose: its role in the treatment of diabetes mellitus. Ann Pharmacother 1996; 30 (11): 1255–62
Spengler M, Cagatay M. The use of acarbose in the primarycare setting: evaluation of efficacy and tolerability of acrbose by postmarketing surveillance study. Clin Invest Med 1995; 18 (4): 325–31
Niskanen L. Hyperinsulinemia at onset and during the early years of NIDDM. In: Standl E, editor. Perspectives of the hyperinsulinemia/insulin resistance syndrome in NIDDM. MMW Med Verlag Münch 1990; Special Edition: 49–58
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Banz, K., Dinkel, R., Hanefeld, M. et al. Evaluation of the Potential Clinical and Economic Effects of Bodyweight Stabilisation with Acarbose in Patients with Type 2 Diabetes Mellitus. Pharmacoeconomics 13, 449–459 (1998). https://doi.org/10.2165/00019053-199813040-00007
Published:
Issue Date:
DOI: https://doi.org/10.2165/00019053-199813040-00007