Summary
Synopsis
Cytomegalovirus retinitis, an opportunistic infection caused by the herpesvirus cytomegalovirus, is a major cause of illness in patients with advanced AIDS. As infected patients require long term drug treatment to delay disease progression and minimise loss of vision, the disease is associated with substantial treatment costs which considerably increase overall expenditure on AIDS-related health care.
During the last decade, intravenous ganciclovir has been a mainstay of treatment for patients with cytomegalovirus retinitis. However, notwithstanding its demonstrated efficacy as maintenance therapy for this condition, long term intravenous drug administration is both inconvenient and uncomfortable for many patients. Moreover, neutropenia and catheter-related infections have been reported commonly in patients receiving ganciclovir via the intravenous route. To overcome the limitations of intravenous ganciclovir, an oral formulation of the drug has been developed for use as maintenance therapy. In comparative clinical trials, both intravenous and oral ganciclovir maintenance therapy slowed disease progression and preserved visual acuity in patients with stabilised cytomegalovirus retinitis, although there was evidence that the intravenous formulation was more effective in terms of delaying recurrence of active disease. This suggests that oral ganciclovir use should be limited to the treatment of patients without evidence of immediately sight-threatening cytomegalovirus retinitis.
Three published cost analyses, which were based on efficacy and tolerability data derived from 2 randomised, comparative clinical trials, have shown that oral ganciclovir maintenance therapy offers cost advantages over intravenous maintenance therapy, despite the higher acquisition cost of the oral formulation. The higher overall costs of intravenous maintenance treatment, compared with oral therapy, were attributed to higher drug administration and adverse event treatment costs. In one analysis, estimated lifetime treatment costs of oral maintenance therapy were 25.2% lower than those of intravenous maintenance treatment. As yet, no formal cost-effectiveness evaluations of oral and intravenous ganciclovir have been published.
Few published data are available regarding the relative effects of intravenous and oral ganciclovir on quality of life. However, in a health state utility analysis, there was a large overall preference among HIV-infected individuals for oral over intravenous maintenance treatment.
In conclusion, oral ganciclovir appears to be a cost-saving and patient-preferred alternative to its intravenous counterpart for the maintenance therapy of AIDS patients with stabilised cytomegalovirus retinitis in whom there is no evidence of sight-threatening disease.
Overview of Cytomegalovirus Retinitis
The most common manifestation of cytomegalovirus infection in patients with AIDS is cytomegalovirus retinitis, a disease that leads to visual deterioration and eventual blindness in untreated patients. Currently, more than 20 million individuals worldwide are estimated to be infected with HIV. Although it has been suggested that up to 45% of these individuals will eventually develop cytomegalovirus retinitis, this percentage may well be an overestimate for HIV-infected individuals in developing countries.
The incidence of AIDS-related cytomegalovirus retinitis has gradually increased over the last decade, as a result of improved antiretroviral drug therapy and better management of opportunistic infections. These factors contributed to improved survival and resulted in a larger population of patients with advanced AIDS at high risk of developing cytomegalovirus retinitis. However, a decrease in the incidence of cytomegalovirus retinitis has recently become evident. This is probably due to the use of highly effective 2- and 3-drug combinations of antiretroviral agents.
Intravenous ganciclovir has been established for several years as a treatment for patients with AIDS-related cytomegalovirus retinitis. It is initially administered as induction therapy (generally as 5 mg/kg twice daily for 2 to 3 weeks) to stop retinal lesion progression and then as long term maintenance therapy in a dosage of 5 mg/kg/day. Recently, oral ganciclovir (3000 mg/day, administered in 3 or 6 divided doses after food) has also been approved as maintenance therapy.
Other agents used to treat patients with cytomegalovirus retinitis include intravenous foscarnet and cidofovir. As yet, no formal pharmacoeconomic evaluations have compared these agents with intravenous or oral ganciclovir.
Pharmacoeconomic Considerations in the Treatment of Cytomegalovirus Retinitis
Patients with cytomegalovirus retinitis require costly, lifelong maintenance therapy to slow progression of retinal lesions and to minimise loss of vision. Treatment costs of this disease have been reported to substantially increase overall expenditure on AIDS-related health care.
In addition to the acquisition costs of drug therapy, direct costs incurred in the treatment of cytomegalovirus retinitis include drug administration costs, which vary according to the route used, and costs associated with the management of treatment-related adverse events. Other direct healthcare costs include those of nursing time and physician consultations.
The maintenance treatment of patients with cytomegalovirus retinitis also involves several nonmedical direct and indirect societal costs. Among these are the costs of work absence and lost productivity, as well as travel expenses. However, these costs may not have a significant effect on overall costs of treatment, given that cytomegalovirus retinitis generally occurs in patients with advanced AIDS who are no longer able to work.
Rationale for use of Ganciclovir in Cytomegalovirus Retinitis
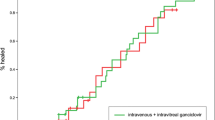
Both intravenous and oral ganciclovir have demonstrated efficacy as maintenance therapy for patients with stabilised AIDS-related cytomegalovirus retinitis. In 2 major comparative clinical trials, assessments of the primary efficacy end-point of time to disease progression by masked assessment of retinal photographs showed no significant advantage for intravenous over oral ganciclovir therapy. Assessments by unmasked funduscopy, reflecting typical clinical practice but subject to observer bias, revealed a significant efficacy advantage for intravenous over oral ganciclovir in terms of time to disease progression.
Neutropenia and thrombocytopenia have been reported as the most common adverse events in patients receiving intravenous ganciclovir. In 2 comparative trials, neutropenia occurred in 23 and 37% of patients on intravenous therapy, compared with 14 and 24% of patients who received the oral formulation. In the same trials, sepsis was reported in 8.5 and 19% of intravenous ganciclovir recipients compared with 3 and 8% of patients who received the oral formulation.
Pharmacoeconomic Evaluation of Ganciclovir
Cost analyses conducted using data from the US, UK and Germany have shown that direct healthcare costs of oral ganciclovir maintenance therapy are generally lower than those of intravenous maintenance therapy. Models, based on efficacy and tolerability data from comparative clinical trials and nontrial resource usage data, were used to assess the economic effect of both types of treatment. All cost analyses were conducted from the perspective of the healthcare payer.
In a US evaluation, which examined the direct costs of treatment from the perspective of the state Medicaid payer, costs of intravenous induction and maintenance therapy were included in the main analysis, whereas reinduction treatment costs were not estimated. On the basis of photographic assessments of clinical efficacy (57 and 62 days free of disease progression for oral and intravenous treatment, respectively) direct healthcare costs of oral ganciclovir treatment were estimated to be about 42% lower than those of intravenous treatment [$US4938 vs $US8587 (1993 dollars)]. In addition, compared with intravenous ganciclovir, estimated lifetime treatment costs (which included estimates of reinduction costs) were 25.2% lower for the oral formulation ($US25 007 vs $US33 453), based on photographic assessments. Sensitivity analysis indicated that oral ganciclovir remained less costly than the intravenous drug when funduscopy data were substituted for photographic data.
Oral ganciclovir treatment was also associated with substantially lower direct medical care costs than intravenous therapy in a German economic evaluation that was based on efficacy and tolerability data from the Oral Ganciclovir European and Australian Cooperative Study. Maintenance and reinduction nondrug treatment costs were included, but first induction and maintenance drug acquisition costs were excluded. Estimated direct costs of intravenous induction in the hospital (for inpatients) and intravenous maintenance therapy administration in the physician’s office or at home were DM17 448 and DM20 284 (in 1993 currency), based on photographic data. Lower costs were estimated for intravenous induction in the hospital plus oral maintenance therapy; these costs were, respectively, DM14 438 and DM8609, based on data from photographic or funduscopic assessments. The lower estimated costs of oral therapy were attributed to the avoidance of costs associated with long term intravenous administration, such as placement of a central venous catheter. Sensitivity analyses showed that the results of the primary cost analysis were robust to a wide range of independent variables, including different adverse event probabilities, and different mean times to disease progression.
Expected costs of oral ganciclovir maintenance therapy were also lower than those of intravenous maintenance treatment in an evaluation conducted in the UK. As in the German study, drug acquisition costs were not available for inclusion in this model, but both reinduction and induction costs were incorporated. In the main analysis, the mean total expected cost per patient was 9% lower with oral therapy than with intravenous maintenance therapy [£7414 vs £8147 (in 1993/94 currency)]. Subsequent application of drug acquisition cost data for maintenance therapy to the model showed that oral ganciclovir was less costly than intravenous ganciclovir maintenance therapy when high unit costs of resources or home intravenous infusion pumps were used; however, for all other scenarios total costs of oral treatment were estimated to be £126 per patient to £1350 per patient higher than intravenous treatment. According to the investigators, underestimates of intravenous treatment costs may have been used in this study.
A health state utility analysis assessed differences in patient preference for the 2 formulations and showed that most interviewed patients with HIV infection would prefer treatment with oral ganciclovir to intravenous treatment in the event of their developing cytomegalovirus retinitis.
A survey of ganciclovir resource utilisation and treatment patterns in 5 European countries showed that protocols for intravenous maintenance therapy varied widely between countries. On the basis of findings of this investigation, it was concluded that the use of oral ganciclovir maintenance therapy had the potential to reduce healthcare resource use in the countries surveyed.
Similar content being viewed by others
References
Jabs DA. Treatment of cytomegalovirus retinitis in patients with AIDS. Ann Intern Med 1996 Jul 15; 125: 144–5
Gallant JE, Moore RD, Richman DD, et al. Incidence and natural history of cytomegalovirus disease in patients with advanced human immunodeficiency virus disease treated wtih zidovudine. J Infect Dis 1993; 166: 1223–7
Gable CB, Tierce JC, Simison D, et al. Costs of HIV+/AIDS at CD4+ counts disease stages based on treatment protocols. J Acquir Immune Defic Syndr 1996; 12(4): 413–20
Sullivan SD, Mozaffari E, Johnson ES, et al. An economic evaluation of oral compared with intravenous ganciclovir for maintenance treatment of newly diagnosed cytomegalovirus retinitis in AIDS patients. Clin Ther 1996 May–Jun; 18: 546–58
Markham A, Faulds D. Ganciclovir: an update of its therapeutic use in cytomegalovirus infection. Drugs 1994 Sep; 48: 455–84
Faulds D, Heel RC. Ganciclovir: a review of its antiviral activity, pharmacokinetic properties and therapeutic efficacy in cytomegalovirus infections. Drugs 1990; 39: 597–638
Holland GN, Tufail A. New therapies for cytomegalovirus retinitis. N Engl J Med 1995 Sep 7; 333: 658–9
Wu AW, Coleson LC, Holbrook J, et al. Measuring visual function and quality of life in patients with cytomegalovirus retinitis. Development of a questionnaire. Studies of Ocular Complication of AIDS Research Group. Arch Ophthalmol 1996 Jul; 114: 841–7
Polis MA, Masur H. Promising new treatments for cytomegalovirus retinitis. JAMA 1995 May 10; 273: 1457–9
Martin DF, Parks DJ, Mellow SD, et al. Treatment of cytomegalovirus retinitis with an intraocular sustained-release ganciclovir implant. A randomized controlled clinical trial. Arch Ophthalmol 1994; 112: 1531–37
Hardy WH. Management strategies for patients with cytomegalovirus retinitis. J Acquir Immune Defic Syndrom Hum Retrovirol 1997; 14 Suppl. 1: S7–S12
Kuppermann BD, Petty JG, Richman DD, et al. Correlation between CD4+ counts and prevalence of cytomegalovirus retinitis and human immunodeficiency virus-related noninfectious retinal vasculopathy in patients with acquired immunodeficiency syndrome. Am J Ophthalmol 1993; 115: 575–82
Shafran SD, Conly JM. Treatment of cytomegalovirus retinitis: a growing number of options. Can J Infect Dis 1996 Nov–Dec; 7: 353–5
Freeman WR. New developments in the treatment of CMV retinitis [editorial; comment]. Ophthalmology 1996 Jul; 103: 999–1000
Hoover DR, Saah AJ, Bacellar H, et al. Clinical manifestations of AIDS in the era of Pneumocystis prophylaxis. Multicenter AIDS Cohort Study. Ann Intern Med 1993; 329: 1922–6
Luckie AP. Cytomegalovirus retinitis in patients with AIDS: current status. Med J Aust 1995 Nov 6; 163: 489–92
Masur H, Whitcup SM, Cartwright C, et al. Advances in the management of AIDS-related cytomegalovirus retinitis. Ann Intern Med 1996 Jul 15; 125: 126–36
Drew WL, Ives D, Lalezari JP, et al. Oral ganciclovir as maintenance treatment for cytomegalovirus retinitis in patients with AIDS. N Engl J Med 1995 Sep 7; 333: 615–20
Luckie A, Ai E. Diagnosis and management of cytomegalovirus retinitis in AIDS. Curr Opin Ophthalmol 1993 Jun; 4: 81–9
Cohn JA. HIV-infection-I. BMJ 1997 Feb 15; 314: 487–91
Studies of Ocular Complications of AIDS Research Group, AIDS Clinical Trials Group. Combination foscarnet and ganciclovir therapy vs monotherapy for the treatment of relapsed cytomegalovirus retinitis in patients with AIDS: the Cytomegalovirus Retreatment Trial. Arch Ophthalmol 1996 Jan; 114: 23–33
Studies of Ocular Complications of AIDS Research Group, AIDS Clinical Trials Group. Mortality in patients with the acquired immunodeficiency syndrome treated with either foscarnet or ganciclovir for cytomegalovirus retinitis. N Engl J Med 1992 Jan 23; 326: 213–20
Jabs DA. Controversies in the treatment of cytomegalovirus retinitis: foscarnet versus ganciclovir. Infect Agents Dis 1995 Sep; 4: 131–42
Lea AP, Bryson HM. Cidofovir. Drugs 1996; 52(2): 225–30
The Oral Ganciclovir European and Australian Cooperative Study Group. Intravenous versus oral ganciclovir: European/Australian comparative study of efficacy and safety in the prevention of cytomegalovirus retinitis recurrence in patients with AIDS. AIDS 1995; 9(5): 471–7
Davies L, Maynard A. An economic exploration of oral and intravenous ganciclovir in the induction and maintenance treatment of AIDS-related cytomegalovirus retinitis. Int J STD AIDS 1996; 7: 415–21
Hellinger FJ, Fleishman JA, Hsia DC. AIDS treatment costs during the last months of life: evidence from the ACSUS. Health Serv Res 1994; 29: 569–81
von den Schulenburg J-MG, Wähling S, Stoll M. German health economic cost evaluation on oral ganciclovir in treating cytomegalovirus retinitis. Pharmacoeconomics 1996; 10(5): 522–30
Jacobson MA, Stanley HD, Heaerd SE. Ganciclovir with recombinant methionyl human granulocyte colony-stimulating factor for treatment of cytomegalovirus disease in AIDS patients. AIDS 1992; 6: 515–6
Jacobson MA. Current management of cytomegalovirus disease in patients with AIDS. AIDS Res Hum Retroviruses 1994 Aug; 10: 917–23
Hardy WD. Combined ganciclovir and recombinant human granulocyte-macrophage colony-stimulating factor in the treatment cytomegalovirus retinitis in AIDS patients. J AIDS 1991; 4 Suppl. 1: S22–8
Hardens M. Ganciclovir evaluation in AIDS patients with cytomegalovirus retinitis: a European study of treatment patterns and resource utilization. AIDS 1996 Dec; 10 Suppl. 4: S25–30
Mozaffari E, Sullivan SD. Home care reimbursement for intravenous ganciclovir therapy. Am J Health System Pharm 1996 Jan 15; 53: 161–3
Jabs DA, Dunn JP, Enger C, et al. Cytomegalovirus retinitis and viral resistance: prevalence of resistance at diagnosis, 1994. Arch Ophthalmol 1996 Jul; 114: 809–14
Drew WL, Miner RC, Busch DF, et al. Prevalence of resistance in patients receiving ganciclovir for serious cytomegalovirus infection. J Infect Dis 1991 Apr; 163: 716–9
Anderson RD, Gains GK, Jung D, et al. Ganciclovir absolute bioavailability and steady-state pharmacokinetics after oral administration of two 3000-mg/d dosing regimens in human immunodeficiency virus- and cytomegalovirus-seropositive patients. Clin Ther 1995 May–Jun; 17: 425–32
Plotkin SA, Drew WL, Felsenstein D, et al. Sensitivity of clinical isolates of human cytomegalovirus to 9-(1,3-dihdroxy-2-propoxymethyl)guanine. J Infect Dis 1985; 152: 833–4
Spector SA, Busch DF, Follansbee S, et al. Pharmacokinetic, safety, and antiviral profiles of oral ganciclovir in persons infected with human immunodeficiency virus: a phase I/II study. J Infect Dis 1995 Jun; 171: 1431–7
Squires KE. Oral ganciclovir for cytomegalovirus retinitis in patients with AIDS: results of two randomized studies. AIDS 1996 Dec; 10 Suppl. 4: 13–8
Knospe V, Katlama C, Rozenbaum W, et al. A randomized controlled study of the efficacy and safety of maintenance treatment with IV and oral ganciclovir in the prevention of recurrence of cytomegalovirus retinitis in AIDS patients [abstract]. 9th International Conference on AIDS. 1993 Jun 6–11; Berlin, 345
Spector SA, Weingeist T, Pollard RB, et al. A randomized, controlled study of intravenous ganciclovir therapy for cytomegalovirus peripheral retinitis in patients with AIDS. J Infect Dis 1993 Sep; 168: 557–63
DeArmond B. Safety considerations in the use of ganciclovir in immunocompromised patients. Transplant Proc 1991; 23 Suppl. 1: 26–29
Johnson ES, Sullivan SD, Mozaffari E, et al. A utility assessment of oral and intravenous ganciclovir for the maintenance treatment of AIDS-related cytomegalovirus retinitis. Pharmacoeconomics 1996 Dec; 10: 623–9
Benefit Research Group Syntex. Economic evaluation of oral ganciclovir versus IV ganciclovir. Roche Pharmaceuticals, I/SYGA/1, 1994. Data on file
Benefit Research Group Syntex. Economic evaluation of oral ganciclovir versus IV ganciclovir in The Netherlands Roche Pharmaceuticals, I/SYGA/1, 1995. Data on file
Froberg DG, Kane RL. Methodology for measuring health-state preferences-III: population and context effects. J Clin Epidemiol 1989; 42(6): 585–92
Anand R, Font RL, Fish RH, et al. Cost of sustained release treatment of CMV retinitis. Reply [letter]. Ophthalmology 1993 Nov; 100: 1603
van der Meer JTM, Drew WL, Bowden RA, et al. Summary of the International Consensus Symposium on Advances in the Diagnosis, Treatment and Prophylaxis of Cytomegalovirus Infection. Antiviral Res 1996 Nov; 32: 119–40
Author information
Authors and Affiliations
Corresponding author
Additional information
Various sections of the manuscript reviewed by: W.L. Drew, University of California, Mt Zion Medical Center, San Francisco, California, USA; M. Hardens, The Lewin Group, Leiden, The Netherlands; F.J. Hellinger, Agency For Health Care Policy and Research, Rockville, Maryland, USA; D.A. Jabs, The Johns Hopkins University and Hospital, The Wilmer Ophthalmic Institute, Baltimore, Maryland, USA; M.A. Jacobson; University of California at San Francisco AIDS Program, San Francisco General Hospital, San Francisco; California, USA; E.S. Johnson, Epidemiology Resources, Inc., Newton Lower Falls, Massachusetts, USA; P.C. Langley, Center for Pharmaceutical Economics, College of Pharmacy, University of Arizona, Tucson, Arizona, USA; A.P. Luckie, Melbourne, Victoria, Australia; E. Mozaffari, Palo Alto, California, USA; S.R. Norrby, University of Lund, Department of Infectious Diseases, University Hospital of Lund, Lund, Sweden; S.B. Palmer, Centre for Health Economics, University of York, York, England; J-M.G. von den Schulenburg, University of Hannover, North German Center for Health Services Research, Hannover, Germany; M. Stoll, Department of Clinical Immunology, Medical School of Hannover, Hannover, Germany; S.D. Sullivan, School of Pharmacy, University of Washington, Health Sciences Center, Seattle, Washington, USA.
Rights and permissions
About this article
Cite this article
Perry, C.M., Davis, R. Ganciclovir. Pharmacoeconomics 12, 209–228 (1997). https://doi.org/10.2165/00019053-199712020-00010
Published:
Issue Date:
DOI: https://doi.org/10.2165/00019053-199712020-00010