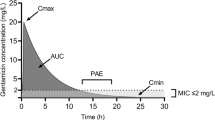
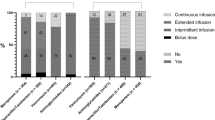
Summary
A fuller understanding of the pharmacodynamics of aminoglycoside antibiotics now exists compared with when they were introduced. Recent findings have shown that once-daily dosage regimens of aminoglycosides are as effective as bd or tid regimens in the Treatment of Gram-negative sepsis. However, radical changes in dosage frequency based on this knowledge are resisted by some physicians because of fears about the peak concentration Toxicity of aminoglycosides These fears have been shown to be misplaced. The delay in the translation of research findings into practice may be attributable to the sheer quantity of medical literature and the limited time that clinicians have available to read it.
Because healthcare resources are finite, physicians arc increasingly becoming aware of the need to use drug therapy in the most cost-effective way. An important component of amino glycoside therapy that may persuade clinicians to change their practice is the organised consideration of the various costs associated with different administration regimens. This review examines the source of those costs, and endorses once-daily dosage of aminoglycosides from both an economic and practical viewpoint.
Similar content being viewed by others
References
Karlowsky JA, Zhanel GG, Davidson RJ. et al. Postantibiotic effect in Pseudomonas aeruginsa following single and multiple aminoglycoside exposures in vitro. J Antimicrob Chem other 1994; 33: 937–47
Daikos GL, Jackson GG, Lolans VT, et al. Adaptive resistance to aminoglycoside antibiotics from first–exposure down–regulation. J Infect Dis 1990; 162: 414–20
Daikos GL, Lolans VT, Jackson GG. First–exposure adaptive resistance to aminoglycoside antibiotics in vivo with meaning for optimal clinical use. Antimicrob Agents Chemother 1991; 35 (1): 117–23
Prins JM, Buller HR, Kuijper J, et al. Once versus thrice daily gentamicin in patients with serious infections. Lancet 1993 Feb 6; 341: 335–9
Noone P, Parsons TMC, Pattison JR, et al. Experience in monitoring gentamicin therapy during treatment of various gram–negative sepsis. BMJ 1974 Mar 16; 1: 477–81
Shrimpton SB, Milmoc M, Wilson APR, et al. Audit of prescription and assay of aminoglycosides in a UK teaching hospital. J Antimkrob Chemother 1993; 31: 599–606
Li SC, Loannides-Demos W, Spicer WJ, et al. Prospective audit of aminoglycoside usage in a general hospital with assessments of clinical processes and adverse clinical outcomes. Med J Aust 1989: 151: 224–32
Flint LM, Gott J, Short L, et al. Serum level monitoring of aminoglycoside antibiotics: limitations in intensive care unit related bacterial pneumonia. Arch Surg 1985; 120: 99–103
Summer WR, Michael JR, Lipsky JJ. Initial aminoglycoside levels in the critically ill. Crit Care Med 1983: 11 (12): 948–50
Zeitany KG, El Saghir NS, Santhosh-Kumar CHR. et al. Increased aminoglycoside dosage requirements in hematologic malignancy. Antimicrob Agents Chemother 1990; 34: 702–8
Claiborne Dunagan W, Woodward RS, Medoff G, et al. Antibiotic misuse in two clinical situations: positive blood culture and administration of aminoglycosides. Rev Infect Dis 1991: 13: 405–12
Jackson GG, Riff LJ. Pseudomonas bacteremia: phatmacologic and other bases for failure of treatment with gentamicin. J Infect Dis 1971: 124 Suppl.124: 5185–91
Black RE, Lau WK, Weinstein RJ, et al. Ototoxicity of amikacin. Antimicrob Agents Chemother 1976; 9: 956–61
Mattie H, Craig WA, Pechere JC. Determinants of efficacy and toxicity of aminoglycosides. J Anti microb Chemother 1989; 24: 281–93
Verpooten GA, Giuliano RA, Verbist L, et al. Once daily dosing decreases renal accumulation of gentamicin and netilmicin. Clin Pharmacol Ther 1989: 45 (1): 22–7
Hollender LF, Bahini J, De Manzini N, et al. A multicentric study of netilmicin once daily versus thrice daily in patients with appendicitis and other intra–abdominal infections. J Antimicrob Chemother 1989; 23: 773–83
Nordstrom L, Ringber H, Cronberg S, et al. Does administration of an aminoglycoside in a single daily dose affect its efficacy and toxicity. J Antimicrob Chemother 1990: 25; 159–73
EORTC International Antimicrobial Therapy Cooperative Group. Single v. multiple daily doses of amikacin combined with ceftriaxone or ceftazidime for empirical therapy of fever in granulocytopenic cancer patients. Ann Intern Med 1993; 119: 584–93
De Vries PJ, Verkooyen RP, Leguit P, et al. Prospective raoom filed study of once daily versus thrice daily nctilmicitt regimens in patients with intra abdominal infections. Eur J Microbiol lnfect Dis 1990: 9: 161–8
Sturm AW. Effects of a restrictive antibiotic policy on clinical efficacy of antibiotics and susceptibility patterns of organisms. Eur J Clin Microbiol Infect Dis 1990; 9: 381–9
Ter Braak EW, De Vries PJ, Bouter KP, et al. Once daily dosing regimen for aminoglycosides plus βlactam combination therapy of serious bacterial infections: comparative trial with netilmicin plus ceftriaxone. Am J Med 1990: 89: 58–66
Moore RD, Lietman PS, Smith CR. Clinical response to aminoglycoside therapy: importance of the ratio of peak concentration to minimal inhibitory concentration. J Infect Dis 1987; 155: 93–9
Rozdzinski E, Kern WV, Reichle A, et al. Once daily versus, thrice daily dosing of netilmicin in combination with βlactam antibiotics as empirical therapy for febrile neutropenic patients. J Antimicrob Chemother 1993: 31: 585–98
Janknegt R. Aminoglycoside monitoring in the once–or twice daily era. Pharm World Sci 1993; 15 (4): 151–5
Peters DA. Implementation of research findings. Health Bull 1992; 50: 68–77
Evidence–Based Care Research Group. Evidence–based care: I. Setting priorities: how important is this problem? Can Med Assoc J 1994: 150 (8): 1249–53
Parker SE, Davey PG. Practicalities of once–daily aminoglycoside dosing. J Antimicrob Chemother 1993; 31: 4–8
Parker SE, Davey PG. Once daily aminoglycoside dosing. Lancet 1993: 341: 346–7
Davey P, Dodd T, Kerr S, et al. Audit of iv antibiotic administration. Pharm J 1990 Jun 30: 244: 793–6
Gyssens IC, van der Meer JWM, Lennards CA, et al. Cost of hospital antimicrobial chemotherapy. A method for global, cost calculation. Pharm Weekbl Sci 1991: 13: 248–53
Weinstein MC, Read JL, MacKay DN, et al. Cost effective choice of antimicrobial therapy for serious infections. J Gen Intern Med 1986 Nov/Dec: 1: 351–63
Plumridge RJ. Cost comparison of intravenous antibiotic administration. Med J Aust 1990: 153: 516–8
Tanner DJ, Nazarian MQ. Cost containment associated with decreased parenteral amibiotic administration frequencies. Am J Med 1984; 77 Suppl. 4C: 104–10
Parker SE, Davey PC. Pharmacoeconomics of intravenous drug administration. Pharmaroeconomics 1992: 1: 103–15
Birdwell SW, Meyer GE, Scheckelhoff DJ, et al. Survey of wastage from intravenous admixture in US hospitals. Pharmacoeconomics, 1993: 4: 271–7
Birdwell SW, Direct costs of intravenous delivery systems. Pharmacoeconomics 1993: 4 (1): 8–13
Vacani PF, Malek MMH, Davey PG, Cost of gentamicin assays carried out by microbiology laboratories. J Clin Pathol 1993; 46: 890–5
D’Angio RG, Stevenson JG, Lively BT, et al. Therapeutic drug monitoring: improved performance through educational intervention. Drug Monit 1990: 12: 173–81
Zaske E, Cipolle RJ, Sirate RJ. Gentamicin dosage requirements: wide interpatient variations in 242 surgery patients with normal renal function. Surgery 1980; 87: 164–9
Davey PG, Geddes AM, Gonda I, et al. Clinical experience with a method for adjusting gentamicin dose from measured drug clearance. J Antimicrob Chemother 1983; 12: 613–22
Nicolau D, Quintiliani R, Nightingale CH. Once daily gentamicin. Conn Med 1992: 56: 561–3
De Broe M, Giuliano RA, Verpooten GA, Choice of drug and dosage regimen: two important risk factors for aminoglycoside nephrotoxicity. Am J Med 1986: 80 Suppl. 6B: 115–8
Moore RD, Smith CR, Lipsky JJ, et al. Risk factors for nephrotoxicity in patients treated with aminoglycosides. Ann Intern Med 1984: 100: 352–7
Gatell JM, Ferran F, Araujo V, et al. Univariate and multivariate analyses of risk factors predisposing to auditory toxicity in patients receiving aminoglycosides. Antimicrob Agent Chemother 1987; 31 (9): 1383–7
Jackson GG, Arcieri G, Ototoxicity of gentamicin in man: a survey and controlled analysis of clinical experience in the United States. J Infect Dis 1971; 124 Suppl.: 5130–7
Matz GJ. Aminoglycoside Ototoxicity. Am J Otolaryngol 1986: 7: 117–9
Kahlmeter G, Dahlager JI, Aminoglycoside toxicity–a review of clinical studies published between 1975 and 1982. J Antimicrob Chemother 1984: 13 Suppl. A: 9–22
Eisenberg JM, Koffer H, Glick HA, et al. What is the cost of nephrotoxicity associated with aminoglycosides? Ann Intern Med 1987; 107: 900–9
Ramsden RT, Ackrill P, Bobbing oscillopsia from gentamicin toxicity. Br J Audiol 1982: 16: 147–50
Berg K. The toxic effect of streptomycin on the vestibular and cochlear apparatus. Acta Otolaryngol 1951; 97 Suppl. 1; 1–47
Federspil P, Drug–induced sudden hearing loss and vestibular disturbances. Adv Otorhinolaryngol 1981; 27: 144–58
Dayal VS, Smith EL, McCain WG, Cochlear and vestibular gentamicin toxicity. Arch Otolaryngol 1974: 100: 338–40
Duncan R, Melville ID, Severe rombergism due to gentamicin toxicity [letter]. BMJ 1987: 295: 1141
Brahams D. Dosage of gentamicin and monitoring of blood levels: an action fails. Lancet 1986: 1: 1395–6
Davey P, Hernanz C, Lynch W, et al. Human and non–financial costs of hospital–acquired infection. J Hosp Infect 1991: 18 Suppl. A: 79–84
Brushwood DB. Government liable for failure to monitor a patients serum gentamicin in an army hospital. Am J Hosp Pharm 1992: 49: 1748–50
Hencke D, £1/2m for boys made deaf by antibiotic. The Guardian 1984 Jan 13: Sect. Home News: 3 (col. 1)
Zenz C. Occupational medicine. Chicago: Yearbook Medical Publishers, 1975
Lightfoot L, Rogers L, Hundreds killed by doctors relying on outdated manuals. Sunday Times (Scottish Edition) 1995 Feb 2; Sect. 1; 1 (col. 4-7)
Moore RD, Smith CR, Lietman PS. Association of aminoglycoside plasma levels with therapeutic outcome in gram–negative pneumonia. Am J Med 1984: 77: 657–62
Mosdell DM, Morris DM, Voltura A, et al. Antibiotic treatment for surgical peritonitis. Ann Surg 1991; 214 (5): 543–52
Gransden WR, Eykyn SJ, Phillips L, et al. Bacteraemia due to E. coli: a study of 861 episodes. Rev Infect Dis 1990: 12 (6): 1008–18
John JF. What price success? The continuing saga of the toxic: therapeutic ratio in the use of aminoglycoside antibiotics. J lnfect Dis 1988: 158: 1–6
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Parker, S.E., Davey, P.G. Once-Daily Aminoglycoside Administration in Gram–Negative Sepsis. Pharmacoeconomics 7, 393–402 (1995). https://doi.org/10.2165/00019053-199507050-00004
Published:
Issue Date:
DOI: https://doi.org/10.2165/00019053-199507050-00004