Abstract
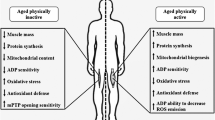
The physiopathology of diabetes mellitus has been closely associated with a variety of alterations in mitochondrial histology, biochemistry and function. Generally, the alterations comprise increased mitochondrial reactive oxygen and nitrogen species (RONS) generation, resulting in oxidative stress and damage; decreased capacity to metabolize lipids, leading to intramyocyte lipid accumulation; and diminished mitochondrial density and reduced levels of uncoupling proteins (UCPs), with consequent impairment in mitochondrial function. Chronic physical exercise is a physiological stimulus able to induce mitochondrial adaptations that can counteract the adverse effects of diabetes on muscle mitochondria. However, the mechanisms responsible for mitochondrial adaptations in the muscles of diabetic patients are still unclear. The main mechanisms by which exercise may be considered an important non-pharmacological strategy for preventing and/or attenuating diabetes-induced mitochondrial impairments may involve (i) increased mitochondrial biogenesis, which is dependent on the increased expression of some important proteins, such as the ‘master switch’ peroxisome proliferator-activated receptor (PPAR)-γ-coactivator-1α (PGC-1α) and heat shock proteins (HSPs), both of which are severely downregulated in the muscles of diabetic patients; and (ii) the restoration or attenuation of the low UCP3 expression in skeletal muscle mitochondria of diabetic patients, which is suggested to play a pivotal role in mitochondrial dysfunction.
There is evidence that chronic exercise and lifestyle interventions reverse impairments in mitochondrial density and size, in the activity of respiratory chain complexes and in cardiolipin content; however, the mechanisms by which chronic exercise alters mitochondrial respiratory parameters, mitochondrial antioxidant systems and other specific proteins involved in mitochondrial metabolism in the muscles of diabetic patients remain to be elucidated.





Similar content being viewed by others
References
Rahimi R, Nikfar S, Larijani B, et al. A review on the role of antioxidants in the management of diabetes and its complications. Biomed Pharmacother 2005; 59 (7): 365–73
Roglic G, Unwin N, Bennett PH, et al. The burden of mortality attributable to diabetes: realistic estimates for the year 2000. Diabetes Care 2005; 28 (9): 2130–5
International Diabetes Federation. Diabetes atlas. 2nd ed. Brussels: International Diabetes Federation, 2003
Luft R. The development of mitochondrial medicine. Proc Natl Acad Sci USA 1994; 91 (19): 8731–8
Fosslien E. Mitochondrial medicine: molecular pathology of defective oxidative phosphorylation. Ann Clin Lab Sci 2001;31 (1): 25–67
Duchen MR. Roles of mitochondria in health and disease. Diabetes 2004; 53 Suppl.1: 96–102
Cadenas E. Mitochondrial free radical production and cell signaling. Mol Aspects Med 2004; 25 (1-2): 17–26
Brookes PS, Yoon Y, Robotham JL, et al. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol Cell Physiol 2004; 287 (4): 817–33
Brookes PS. Mitochondrial H(+) leak and ROS generation: an odd couple. Free Radic Biol Med 2005; 38 (1): 12–23
Skulachev VP. Mitochondrial physiology and pathology; concepts of programmed death of organelles, cells and organisms. Mol Aspects Med 1999; 20 (3): 139–84
Brown GC, Borutaite V. Nitric oxide inhibition of mitochondrialrespiration and its role in cell death. Free Radic Biol Med 2002; 33 (11): 1440–50
Brown GC, Borutaite V. Nitric oxide, mitochondria, and cell death. IUBMB Life 2001; 52 (3-5): 189–95
Kelley DE, He J, Menshikova EV, et al. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes 2002; 51 (10): 2944–50
Toledo FG, Watkins S, Kelley DE. Changes induced by physical activity and weight loss in the morphology of intermy ofibrillar mitochondria in obese men and women. J Clin En docrinol Metab 2006; 91 (8): 3224–7
Mootha VK, Lindgren CM, Eriksson KF, et al. PGC-1alph responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet 2003; 34 (3): 267–73
Patti ME, Butte AJ, Crunkhorn S, et al. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: potential role of PGC1 and NRF1. Proc Natl Acad Sci USA 2003; 100 (14): 8466–71
Petersen KF, Dufour S, Befroy D, et al. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med 2004; 350 (7): 664–71
Morino K, Petersen KF, Dufour S, et al. Reduced mitochondrial density and increased IRS-1 serine phosphorylation in muscle of insulin-resistant offspring of type 2 diabetic parents. J Clin Invest 2005; 115 (12): 3587–93
Irrcher I, Adhihetty PJ, Joseph AM, et al. Regulation of mitochondrial biogenesis in muscle by endurance exercise. Sports Med 2003; 33 (11): 783–93
Adhihetty PJ, Irrcher I, Joseph AM, et al. Plasticity of skeletal muscle mitochondria in response to contractile activity. Exp Physiol 2003; 88 (1): 99–107
Henriksen EJ. Invited review: effects of acute exercise and exercise training on insulin resistance. J Appl Physiol 2002; 93 (2): 788–96
Roberts CK, Won D, Pruthi S, et al. Effect of a diet and exercise intervention on oxidative stress, inflammation and monocyte adhesion in diabetic men. Diabetes Res Clin Pract 2006; 73 (3): 249–59
Short KR, Vittone JL, Bigelow ML, et al. Impact of aerobic exercise training on age-related changes in insulin sensitivity and muscle oxidative capacity. Diabetes 2003; 52 (8): 1888–96
Ascensao A, Ferreira R, Magalhaes J. Exercise-induced cardioprotection: biochemical, morphological and functional evidence in whole tissue and isolated mitochondria. Int J Cardiol 2007; 117: 16–30
el Midaoui A, Tancrede G, Nadeau A. Effect of physical training on mitochondrial function in skeletal muscle of normal and diabetic rats. Metabolism 1996; 45 (7): 810–6
Baar K. Involvement of PPAR gamma co-activator-1, nuclear respiratory factors 1 and 2, and PPAR alpha in the adaptive response to endurance exercise. Proc Nutr Soc 2004; 63 (2): 269–73
Fritz T, Kramer DK, Karlsson HK, et al. Low-intensity exercise increases skeletal muscle protein expression of PPARdelta and UCP3 in type 2 diabetic patients. Diabetes Metab Res Rev 2006; 22 (6): 492–8
van Loon LJ, Goodpaster BH. Increased intramuscular lipid storage in the insulin-resistant and endurance-trained state. Pflugers Arch 2006; 451 (5): 606–16
Toledo FG, Menshikova EV, Ritov VB, et al. Effects of physical activity and weight loss on skeletal muscle mitochondria and relationship with glucose control in type 2 diabetes. Diabetes 2007; 56 (8): 2142–7
McCarty MF. Up-regulation of PPARgamma coac tivator-1alpha as a strategy for preventing and reversing insulin resistance and obesity. Med Hypotheses 2005; 64 (2): 399–407
Sriwijitkamol A, Ivy JL, Christ-Roberts C, et al. LKB1-AMPK signaling in muscle from obese insulin-resistant Zucker rats and effects of training. Am J Physiol Endocrinol Metab 2006; 290 (5): 925–32
Oliveira PJ, Rolo AP, Seica R, et al. Decreased susceptibility of heart mitochondria from diabetic GK rats to mitochondrial permeability transition induced by calcium phosphate. Biosci Rep 2001; 21 (1): 45–53
Oliveira PJ, Seica R, Santos DL, et al. Vitamin E or coenzyme Q10 administration is not fully advantageous for heart mitochondrial function in diabetic goto kakizaki rats. Mitochondrion 2004; 3 (6): 337–45
Kayali R, Cakatay U, Telci A, et al. Decrease in mitochondrial oxidative protein damage parameters in the streptozotocindiabetic rat. Diabetes Metab Res Rev 2004; 20 (4): 315–21
Shen X, Zheng S, Thongboonkerd V, et al. Cardiac mitochonial damage and biogenesis in a chronic model of type 1 diabetes. Am J Physiol Endocrinol Metab 2004; 287 (5):896–905
Antonetti DA, Reynet C, Kahn CR. Increased expression of mitochondrial-encoded genes in skeletal muscle of humans with diabetes mellitus. J Clin Invest 1995; 95 (3): 1383–8
Sreekumar R, Halvatsiotis P, Schimke JC, et al. Gene expression profile in skeletal muscle of type 2 diabetes and the effect of insulin treatment. Diabetes 2002; 51 (6): 1913–20
Hojlund K, Wrzesinski K, Larsen PM, et al. Proteome analysis reveals phosphorylation of ATP synthase beta-subunit in human skeletal muscle and proteins with potential roles in type 2 diabetes. J Biol Chem 2003; 278 (12): 10436–42
Ritov VB, Menshikova EV, He J, et al. Deficiency of subsarcolemmal mitochondria in obesity and type 2 diabetes. Diabetes 2005; 54 (1): 8–14
Befroy DE, Petersen KF, Dufour S, et al. Impaired mitochondrial substrate oxidation in muscle of insulin-resistant offspring of type 2 diabetic patients. Diabetes 2007; 56 (5): 1376–81
Petersen KF, Befroy D, Dufour S, et al. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science 2003; 300 (5622): 1140–2
He J, Goodpaster BH, Kelley DE. Effects of weight loss and physical activity on muscle lipid content and droplet size. ObesRes 2004; 12 (5): 761–9
Mogensen M, Sahlin K, Fernstrom M, et al. Mitochondrial respiration is decreased in skeletal muscle of patients with type 2 diabetes. Diabetes 2007; 56 (6): 1592–9
Franks PW, Loos RJ. PGC-1alpha gene and physical activity in type 2 diabetes mellitus. Exerc Sport Sci Rev 2006; 34 (4): 171–5
Tanaka T, Yamamoto J, Iwasaki S, et al. Activation of peroxisome proliferator-activated receptor delta induces fatty acid beta-oxidation in skeletal muscle and attenuates metabolic syndrome. Proc Natl Acad Sci USA 2003; 100 (26): 15924–9
Frenzel H, Schwartzkopff B, Holtermann W, et al. Regression of cardiac hypertrophy: morphometric and biochemical studies in rat heart after swimming training. J Mol Cell Cardiol 1988:20 (8): 737–51
Hoppeler H, Fluck M. Plasticity of skeletal muscle mitochondria: structure and function. Med Sci Sports Exerc 2003; 35 (1): 95–10
Hood DA. Invited Review: contractile activity-induced mitochondrial biogenesis in skeletal muscle. J Appl Physiol 2001;90 (3): 1137–57
Hood DA, Adhihetty PJ, Colavecchia M, et al. Mitochondrial biogenesis and the role of the protein import pathway. Med Sci Sports Exerc 2003; 35 (1): 86–94
Ascensão A, Magalhães J. Exercise and mitochondrial function in striated muscle. In: Moreno AJ, Oliveira PJ, Palmeira MC, editors. Mitochondrial pharmacology and toxicology. Kerala: Transworld Research Network, 2006: 237–70
Scarpulla RC. Transcriptional activators and coactivators in the nuclear control of mitochondrial function in mammalian cells. Gene 2002; 286 (1): 81–9
Scarpulla RC. Nuclear activators and coactivators in mammalian mitochondrial biogenesis. Biochim Biophys Acta 2002; 1576 (1-2): 1–14
Bergeron R, Ren JM, Cadman KS, et al. Chronic activation of AMP kinase results in NRF-1 activation and mitochondrial biogenesis. Am J Physiol Endocrinol Metab 2001; 281 (6): 1340–6
Lehman JJ, Barger PM, Kovacs A, et al. Peroxisome proliferator-activated receptor gamma coactivator-1 promotes cardiac mitochondrial biogenesis. J Clin Invest 2000; 106 (7): 847–56
Murakami T, Shimomura Y, Yoshimura A, et al. Induction of nuclear respiratory factor-1 expression by an acute bout of exercise in rat muscle. Biochim Biophys Acta 1998; 1381 (1): 113–22
Xia Y, Buja LM, McMillin JB. Activation of the cytochrome cgene by electrical stimulation in neonatal rat cardiac myocytes:role of NRF-1 and c-Jun. J Biol Chem 1998; 273 (20): 12593–8
Xia Y, Buja LM, Scarpulla RC, et al. Electrical stimulation of neonatal cardiomyocytes results in the sequential activation of nuclear genes governing mitochondrial proliferation and differentiation. Proc Natl Acad Sci USA 1997; 94 (21): 11399–404
Garnier A, Fortin D, Zoll J, et al. Coordinated changes in mitochondrial function and biogenesis in healthy and diseased human skeletal muscle. Faseb J 2005; 19 (1): 43–52
Baar K, Wende AR, Jones TE, et al. Adaptations of skeletal muscle to exercise: rapid increase in the transcriptional coactivator PGC-1. Faseb J 2002; 16 (14): 1879–86
Koves TR, Li P, An J, et al. Peroxisome proliferator-activated receptor-gamma co-activator 1alpha-mediated metabolic remodeling of skeletal myocytes mimics exercise training and reverses lipid-induced mitochondrial inefficiency. J Biol Chem 2005; 280 (39): 33588–98
Bengtsson J, Gustafsson T, Widegren U, et al. Mitochondrial transcription factor A and respiratory complex IV increase in response to exercise training in humans. Pflugers Arch 2001; 443 (1): 61–6
Gordon JW, Rungi AA, Inagaki H, et al. Effects of contractile activity on mitochondrial transcription factor A expression in skeletal muscle. J Appl Physiol 2001; 90 (1): 389–96
Chilibeck PD, Syrotuik DG, Bell GJ. The effect of strength training on estimates of mitochondrial density and distribution throughout muscle fibres. Eur J Appl Physiol Occup Physiol 1999; 80 (6): 604–9
Holten MK, Zacho M, Gaster M, et al. Strength training in creases insulin-mediated glucose uptake, GLUT4 content, and insulin signaling in skeletal muscle in patients with type 2 diabetes. Diabetes 2004; 53 (2): 294–305
Goto M, Terada S, Kato M, et al. cDNA Cloning and mRNA analysis of PGC-1 in epitrochlearis muscle in swimming exercise rats. Biochem Biophys Res Commun 2000; 274 (2): 350–4
Pilegaard H, Saltin B, Neufer PD. Exercise induces transient transcriptional activation of the PGC-1alpha gene in human skeletal muscle. J Physiol 2003; 546 (Pt 3): 851–83
Akimoto T, Pohnert SC, Li P, et al. Exercise stimulates Pgc-1alpha transcription in skeletal muscle through activation of the p38 MAPK pathway. J Biol Chem 2005; 280 (20): 19587–93
Cartoni R, Leger B, Hock MB, et al. Mitofusins 1/2 and ERRalpha expression are increased in human skeletal muscle after physical exercise. J Physiol 2005; 567 (Pt 1): 349–58
Mahoney DJ, Parise G, Melov S, et al. Analysis of global mRNA expression in human skeletal muscle during recovery from endurance exercise. Faseb J 2005; 19 (11): 1498–500
Russell AP, Hesselink MK, Lo SK, et al. Regulation of metabolic transcriptional co-activators and transcription factors with acute exercise. Faseb J 2005; 19 (8): 986–8
Terada S, Kawanaka K, Goto M, et al. Effects of high-intensity intermittent swimming on PGC-1alpha protein expression in rat skeletal muscle. Acta Physiol Scand 2005; 184 (1): 59–65
Adhihetty PJ, Taivassalo T, Haller RG, et al. The effect of training on the expression of mitochondrial biogenesis andapoptosis-related proteins in skeletal muscle of patients with mtDNA defects. Am J Physiol Endocrinol Metab 2007; 293 (3): 672–80
Wright DC, Han DH, Garcia-Roves PM, et al. Exercise-induced mitochondrial biogenesis begins before the increase in muscle PGC-1alpha expression. J Biol Chem 2007; 282 (1): 194–9
Hood DA, Joseph AM. Mitochondrial assembly: protein import. Proc Nutr Soc 2004; 63 (2): 293–300
Hood D, Rungi A, Colavecchia M, et al. Stress proteins and mitochondria. In: Locke M, Noble E, editors. Exercise and stress response: the role of stress proteins. Boca Raton (FL): CRC Press, 2002: 151–62
Takahashi M, Chesley A, Freyssenet D, et al. Contractile activity induced adaptations in the mitochondrial protein import system. Am J Physiol 1998; 274 (5 Pt 1): 1380–7
Ornatsky OI, Connor MK, Hood DA. Expression of stress proteins and mitochondrial chaperonins in chronically stimulated skeletal muscle. Biochem J 1995; 311 (Pt 1): 119–23
Craig EE, Chesley A, Hood DA. Thyroid hormone modifies mitochondrial phenotype by increasing protein import without altering degradation. Am J Physiol 1998; 275 (6 Pt 1): 1508–15
Lennon SL, Quindry JC, French JP, et al. Exercise and myocardial tolerance to ischaemia-reperfusion. Acta Physiol Scand 2004; 182 (2): 161–9
Powers SK, Demirel HA, Vincent HK, et al. Exercise training improves myocardial tolerance to in vivo ischemia-reperfusion in the rat. Am J Physiol 1998; 275 (5 Pt 2): 1468–77
Hamilton KL, Staib JL, Phillips T, et al. Exercise, antioxidants, and HSP72: protection against myocardial ischemia/reperfusion. Free Radic Biol Med 2003; 34 (7): 800–9
Ascensao A, Magalhaes J, Soares J, et al. Endurance training attenuates doxorubicin-induced cardiac oxidative damage in mice. Int J Cardiol 2005; 100 (3): 451–60
Ascensao A, Magalhaes J, Soares JM, et al. Moderate endurance training prevents doxorubicin-induced in vivo mitochondriopathy and reduces the development of cardiac apoptosis. Am J Physiol Heart Circ Physiol 2005; 289 (2): 722–31
Ascensao A, Magalhaes J, Soares JM, et al. Endurance training limits the functional alterations of heart rat mitochondria submitted to in vitro anoxia-reoxygenation. Int J Cardiol 2006; 109 (2): 169–78
Ascensao A, Ferreira R, Oliveira PJ, et al. Effects of endurance chondrial alterations induced by in vitro anoxia-reoxygenation. Cardiovasc Toxicol 2006; 6 (3-4): 159–72
Takahashi M, Hood DA. Chronic stimulation-induced changes in mitochondria and performance in rat skeletal muscle. J Appl Physiol 1993; 74 (2): 934–41
Atalay M, Oksala NK, Laaksonen DE, et al. Exercise training modulates heat shock protein response in diabetic rats. J Appl Physiol 2004; 97 (2): 605–11
Bota DA, Davies KJ. Protein degradation in mitochondria:implications for oxidative stress, aging and disease: a novel etiological classification of mitochondrial proteolytic disorders. Mitochondrion 2001; 1 (1): 33–49
Luciakova K, Sokolikova B, Chloupkova M, et al. Enhanced mitochondrial biogenesis is associated with increased expression of the mitochondrial ATP-dependent Lon protease. FEBS Lett 1999; 444 (2-3): 186–8
Smirnova E, Griparic L, Shurland DL, et al. Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol Biol Cell 2001; 12 (8): 2245–56
Santel A, Frank S, Gaume B, et al. Mitofusin-1 protein is a generally expressed mediator of mitochondrial fusion in mammalian cells. J Cell Sci 2003; 116 (Pt 13): 2763–74
Rojo M, Legros F, Chateau D, et al. Membrane topology and mitochondrial targeting of mitofusins, ubiquitous mammalian homologs of the transmembrane GTPase Fzo. J Cell Sci 2002; 115 (Pt 8): 1663–74
Legros F, Lombes A, Frachon P, et al. Mitochondrial fusion in human cells is efficient, requires the inner membrane potential, and is mediated by mitofusins. Mol Biol Cell 2002; 13 (12): 4343–54
Soriano FX, Liesa M, Bach D, et al. Evidence for a mitochondral regulatory pathway defined by peroxisome proliferator activated receptor-gamma coactivator-1 alpha, estrogen-related receptor-alpha, and mitofusin 2. Diabetes 2006; 55 (6): 1783–91
Bach D, Naon D, Pich S, et al. Expression of Mfn2, the Charcot Marie-Tooth neuropathy type 2A gene, in human skeletal muscle: effects of type 2 diabetes, obesity, weight loss, and the regulatory role of tumor necrosis factor alpha and interleukin-6. Diabetes 2005; 54 (9): 2685–93
Brand MD, Brindle KM, Buckingham JA, et al. The significance and mechanism of mitochondrial proton conductance. Int J Obes Relat Metab Disord 1999; 23 Suppl. 6: 4–11
Ricquier D, Bouillaud F. Mitochondrial uncoupling proteins: from mitochondria to the regulation of energy balance. J Physiol 2000; 529 Pt 1: 3–10
Schrauwen P, Saris WH, Hesselink MK. An alternative function for human uncoupling protein 3: protection of mitochondria against accumulation of nonesterified fatty acids inside the mitochondrial matrix. Faseb J 2001; 15 (13): 2497–502
Himms-Hagen J, Harper ME. Physiological role of UCP3 may be export of fatty acids from mitochondria when fatty acid oxidation predominates: an hypothesis. Exp Biol Med (Maywood) 2001; 226 (2): 78–84
Hesselink MK, Greenhaff PL, Constantin-Teodosiu D, et al. Increased uncoupling protein 3 content does not affect mitochondrial function in human skeletal muscle in vivo. J Clin Invest 2003; 111 (4): 479–86
Hesselink MK, Keizer HA, Borghouts LB, et al. Protein expression of UCP3 differs between human type 1, type 2a, and type 2b fibers. Faseb J 2001; 15 (6): 1071–3
Goglia F, Skulachev VP. A function for novel uncoupling proteins: antioxidant defense of mitochondrial matrix by translocating fatty acid peroxides from the inner to the outer membrane leaflet. Faseb J 2003; 17 (12): 1585–91
Vidal-Puig AJ, Grujic D, Zhang CY, et al. Energy metabolism in uncoupling protein 3 gene knockout mice. J Biol Chem 2000; 275 (21): 16258–66
Korshunov SS, Skulachev VP, Starkov AA. High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett 1997; 416 (1): 15–8
Brand MD, Pamplona R, Portero-Otin M, et al. Oxidative damage and phospholipid fatty acyl composition in skeletal muscle mitochondria from mice underexpressing or overexpressing uncoupling protein 3. Biochem J 2002; 368 (Pt 2): 597–603
Hildebrandt AL, Pilegaard H, Neufer PD. Differential transcriptional activation of select metabolic genes in response to variations in exercise intensity and duration. Am J Physiol variations in exercise intensity and dura Endocrinol Metab 2003; 285 (5): 1021–7
Schrauwen P, Troost FJ, Xia J, et al. Skeletal muscle UCP2 and UCP3 expression in trained and untrained male subjects. Int J Obes Relat Metab Disord 1999; 23 (9): 966–72
Russell A, Wadley G, Snow R, et al. Slow component of [V]O(2) kinetics: the effect of training status, fibre type, UCP3 mRNA and citrate synthase activity. Int J Obes Relat Metab Disord 2002; 26 (2): 157–64
Russell AP, Wadley G, Hesselink MK, et al. UCP3 protein expression is lower in type I, IIa and IIx muscle fiber types of endurance-trained compared to untrained subjects. Pflugers Arch 2003; 445 (5): 563–9
Zhou M, Lin BZ, Coughlin S, et al. UCP-3 expression in skeletal muscle: effects of exercise, hypoxia, and AMP-activated protein kinase. Am J Physiol Endocrinol Metab 2000; 279 (3): 622–9
Pilegaard H, Keller C, Steensberg A, et al. Influence of pre-exercise muscle glycogen content on exercise-induced transcriptional regulation of metabolic genes. J Physiol 2002; 541 (Pt 1): 261–71
Schrauwen P, Hesselink MK, Vaartjes I, et al. Effect of acute exercise on uncoupling protein 3 is a fat metabolism-mediated effect. Am J Physiol Endocrinol Metab 2002; 282 (1): 11–17
Boss O, Samec S, Desplanches D, et al. Effect of endurance training on mRNA expression of uncoupling proteins 1, 2, and 3 in the rat. Faseb J 1998; 12 (3): 335–9
Hjeltnes N, Fernstrom M, Zierath JR, et al. Regulation of UCP2 and UCP3 by muscle disuse and physical activity in tetraplegic subjects. Diabetologia 1999; 42 (7): 826–30
Tonkonogi M, Krook A, Walsh B, et al. Endurance training increases stimulation of uncoupling of skeletal muscle mitochondriain humans by non-esterified fatty acids: an uncoupling- protein-mediated effect? Biochem J 2000; 351 Pt 3: 805–10
Jones TE, Baar K, Ojuka E, et al. Exercise induces an increase in muscle UCP3 as a component of the increase in mitochondrial biogenesis. Am J Physiol Endocrinol Metab 2003; 284 (1): 96–101
Russell AP, Somm E, Praz M, et al. UCP3 protein regulation in human skeletal muscle fibre types I, IIa and IIx is dependent on exercise intensity. J Physiol 2003; 550 (Pt 3): 855–61
Fernstrom M, Tonkonogi M, Sahlin K. Effects of acute and chronic endurance exercise on mitochondrial uncoupling in human skeletal muscle. J Physiol 2004; 554 (Pt 3): 755–63
Schrauwen P, Russell AP, Moonen-Kornips E, et al. Effect of 2 weeks of endurance training on uncoupling protein 3 content in untrained human subjects. Acta Physiol Scand 2005; 183 (3): 273–80
Tsuboyama-Kasaoka N, Tsunoda N, Maruyama K, et al. Upregulation of uncoupling protein 3 (UCP3) mRNA by exercise training and down-regulation of UCP3 by denervation in skeletal muscles. Biochem Biophys Res Commun 1998; 247 (2): 498–503
Cortright RN, Zheng D, Jones JP, et al. Regulation of skeletal muscle UCP-2 and UCP-3 gene expression by exercise and denervation. Am J Physiol 1999; 276 (1 Pt 1): 217–21
Tunstall RJ, Mehan KA, Hargreaves M, et al. Fasting activates the gene expression of UCP3 independent of genes necessary for lipid transport and oxidation in skeletal muscle. Biochem Biophys Res Commun 2002; 294 (2): 301–8
Pilegaard H, Ordway GA, Saltin B, et al. Transcriptional regulationof gene expression in human skeletal muscle during recovery from exercise. Am J Physiol Endocrinol Metab 2000; 279 (4): 806–14
Busquets S, Almendro V, Barreiro E, et al. Activation of UCPs gene expression in skeletal muscle can be independent on both circulating fatty acids and food intake: involvement of ROS in a model of mouse cancer cachexia. FEBS Lett 2005; 579 (3): 717–22
Schrauwen P, Hesselink MK. Oxidative capacity, lipotoxicity, and mitochondrial damage in type 2 diabetes. Diabetes 2004;53 (6): 1412–7
Hesselink MK, Schrauwen P, Holloszy JO, et al. Divergent effects of acute exercise and endurance training on UCP3 expression. Am J Physiol Endocrinol Metab 2003; 284 (2): 449–50; author reply 50-1
Winder WW, Hardie DG. AMP-activated protein kinase, a metabolic master switch: possible roles in type 2 diabetes. Am J Physiol 1999; 277 (1 Pt 1): 1–10
Sriwijitkamol A, Coletta DK, Wajcberg E, et al. Effect of acute exercise on AMPK signaling in skeletal muscle of subjects with type 2 diabetes: a time-course and dose-response study. Diabetes 2007; 56 (3): 836–48
Searls YM, Smirnova IV, Fegley BR, et al. Exercise attenuates diabetes-induced ultrastructural changes in rat cardiac tissue. Med Sci Sports Exerc 2004; 36 (11): 1863–70
Mokhtar N, Lavoie JP, Rousseau-Migneron S, et al. Physical training reverses defect in mitochondrial energy production inheart of chronically diabetic rats. Diabetes 1993; 42 (5): 682–7
Acknowledgements
José A. Lumini and António Ascensão are supported by grants from the Portuguese Foundation for Science and Technology (SFRH/BD/30906/2006 and SFRH/BPD/4225/2007, respectively). The authors have no conflicts of interest that are directly relevant to the content of this review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lumini, J.A., Magalhães, J., Oliveira, P.J. et al. Beneficial Effects of Exercise on Muscle Mitochondrial Function in Diabetes Mellitus. sports med 38, 735–750 (2008). https://doi.org/10.2165/00007256-200838090-00003
Published:
Issue Date:
DOI: https://doi.org/10.2165/00007256-200838090-00003