Abstract
Resistance training can be defined as the act of repeated voluntary muscle contractions against a resistance greater than those normally encountered in activities of daily living. Training of this kind is known to increase strength via adaptations in both the muscular and nervous systems. While the physiology of muscular adaptations following resistance training is well understood, the nature of neural adaptations is less clear. One piece of indirect evidence to indicate that neural adaptations accompany resistance training comes from the phenomenon of ‘cross education’, which describes the strength gain in the opposite, untrained limb following unilateral resistance training. Since its discovery in 1894, subsequent studies have confirmed the existence of cross education in contexts involving voluntary, imagined and electrically stimulated contractions. The crosseducation effect is specific to the contralateral homologous muscle but not restricted to particular muscle groups, ages or genders. A recent meta-analysis determined that the magnitude of cross education is ≈7.8% of the initial strength of the untrained limb. While many features of cross education have been established, the underlying mechanisms are unknown.
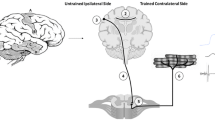
This article provides an overview of cross education and presents plausible hypotheses for its mechanisms. Two hypotheses are outlined that represent the most viable explanations for cross education. These hypotheses are distinct but not necessarily mutually exclusive. They are derived from evidence that highforce, unilateral, voluntary contractions can have an acute and potent effect on the efficacy of neural elements controlling the opposite limb. It is possible that with training, long-lasting adaptations may be induced in neural circuits mediating these crossed effects. The first hypothesis suggests that unilateral resistance training may activate neural circuits that chronically modify the efficacy of motor pathways that project to the opposite untrained limb. This may subsequently lead to an increased capacity to drive the untrained muscles and thus result in increased strength. A number of spinal and cortical circuits that exhibit the potential for this type of adaptation are considered. The second hypothesis suggests that unilateral resistance training induces adaptations in motor areas that are primarily involved in the control of movements of the trained limb. The opposite untrained limb may access these modified neural circuits during maximal voluntary contractions in ways that are analogous to motor learning. A better understanding of the mechanisms underlying cross education may potentially contribute to more effective use of resistance training protocols that exploit these cross-limb effects to improve the recovery of patients with movement disorders that predominantly affect one side of the body.

Similar content being viewed by others
References
Sale DG. Neural adaptation to resistance training. Med Sci Sports Exerc 1988; 20 (5): S135–45
Enoka RM. Neural adaptations with chronic physical activity. J Biomech 1997; 30 (5): 447–55
Enoka RM. Muscle strength and its development: new perspectives. Sports Med 1988; 6: 146–68
Abernethy PJ, Jurimae L, Logan P. Acute and chronic response of skeletal muscle to resistance exercise. Sports Med 1994; 17(1), 22–38
Baldwin KM, Haddad F. Effects of different activity and inactivity paradigms on myosin heavy chain gene expression instriated muscle. J Appl Physiol 2001; 90: 345–57
Scripture EW, Smith n, Brown EM. On the education of muscular control and power. Stud Yale Psychol Lab 1894; 2:114–9
Coleman A. Effect of unilateral isometric and isotonic contraction on the strength of the contralateral limb. Res. Q 1969; 40:490–5
Ikai M, Fukunaga T. A study on training effect on strength per cross-sectional area of muscle by means of ultrasound measurement. Eur J Appl Physiol 1970; 28: 173–80
Moritani T, DeVeries HA. Neural factors versus hypertrophy in the time course of muscle strength gain. Am J Phys Med 1979;58 (3), 115–30
Hakkinen K, Komi PV. Electromyographic changes during strength training and detraining. Med Sci Sports Exerc 1983;15 (6), 455–60
Houston ME, Froese EA, Valeriote SP, et al. Muscle performance, morphology and metabolic capacity during strength training and detraining: a one leg model. Eur J Appl Physiol 1983; 51: 25–35
Yasuda Y, Miyamura M. Cross-transfer effects of muscular training on blood flow in the ipsilateral and contralateral forearms. Eur J Appl Physiol 1983; 51: 321–9
Cannon RJ, Cafarelli E. Neuromuscular adaptations to training. J Appl Physiol 1987; 63 (6): 2396–402
Hortobagyi T, Larmert NJ, Hill JP. Greater cross education following training with muscle lengthening than shortening. Med Sci Sports Exerc 1997; 29: 107–12
Shima N, Ishida K, Katayama K, et al. Cross education of muscular strength during unilateral resistance training and detraining. Eur J Appl Physiol 2002; 86: 287–94
Zhou S. Chronic neural adaptation to unilateral exercise: mechanisms of cross education. Exerc Sports Sci Rev 2000; 28 (4):177–84
Davies CTM, Dooley P, McDonagh MJN, et al. Adaptation of mechanical properties of human to high force training. J Physiol 1985; 365: 277–84
Yue G, Cole KJ. Strength increases from the motor program: comparison of training with maximal voluntary and imagined muscle contractions. J Neurophysiol 1992; 67: 1114–23
Carolan B, Cafarelli E. Adaptations in coactivation after isometric resistance training. J Appl Physiol 1992; 73 (3): 911–7
Ploutz P, Tesch PA, Biro RL, et al. Effect of resistance training on muscle use. J Appl PhysioI 1994; 76 (4): 1675–81
Evetovich T, Housh TJ, Housh DJ, et al. The effects of chronic isokinetic strength training of the quadriceps femoris on electromyographand muscle strength in the trained and untrained limb. J Strength Cond Res 2001; 15 (4): 439–45
Hortobagyi T, Scott K, Larillert NJ, et al. Cross-education of muscle strength is greater with stimulated than voluntary contractions. Motor Control 1999; 3: 205–19
Oakman A, Zhou S, Davie A. Cross-education effect observed in voluntary and electromyo stimulation strength training. In: Sanders RH, Gibson BJ, editors. XVII International Symposium of Biomechanics in Sports; 1999 Jun 30-Jul 6; Perth. Perth(WA): Edith Cowan University, 1999: 401–4
Ranganathan VK, Siemionow V, Liu JZ, et al. From mental power to muscle power-gaining strength by using the mind. Neuropsychologia 2004; 42: 944–56
Devine KL, LeVeau BF, Yack HJ. Electromyographic activity recorded from an unexercised muscle during maximal isometric exercise of the contralateral agonists and antagonists. Phys Ther 1981; 61 (6): 898–903
Narici MV, Roi GS, Landoni L, et al. Changes in cross-sectional area and neural activation during strength training and detrainingof human quadriceps. Eur J Appl Physiol 1989; 59:310–9
Munn J, Herbert RD, Gandevia SC. Contralateral effects of unilateral resistance training: a meta-analysis. J Appl Physiol2004; 96: 1861–6
Herbert RD, Dean C, Gandevia SC. Fifects of real and imagined training on voluntary muscle activation during maximal isometric contractions. Acta Physiol Scand 1998; 163: 361–8
Gleeson NP, Mercer TH. The utility of isokinetic dynamometry in the assessment of human muscle function. Sports Med 1996;21: 18–34
Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev 2001; 81 (4): 1725–89
Munn J, Herbert RD, HancockMJ, et al. Training with unilateral resistance exercise increases contralateral strength. J Appl Physiol 2005; 99 (5): 1880–4
Eklund B, Kaijser L, Knutsson E. Blood flow in resting (contralateral) arm and leg during isometric contraction. J Physiol 1974; 240 (1): 111–24
Eklund B, Kaijser L. Effect of regional alpha- and beta-adrenergic blockade on blood flow in the resting forearm during contralateral isometric hand grip. J Physiol 1976; 262 (1):39–50
Gandevia SC, Allen GM, Butler JE, et al. Supraspinal factors in human muscle fatigue: evidence for sub optimal output from the motor cortex. J Physiol 1996; 490 (Pt 2): 529–36
Gandevia SC. Neural control in human muscle fatigue: changes in muscle afferents, motoneurones and motor cortical drive[corrected]. Acta Physiol Scand 1998; 162 (3): 275–83
Hortobagyi T, Taylor JL, Petersen NT, et al. Changes in segmental and motor cortical output with contralateral muscle contractions and altered sensory inputs in human. J Neurophysiol 2003; 90: 2451–9
Carson RG, Riek S, Mackey DC, et al. Excitability changes in human forearm cortico spinal projections and spinal reflex pathways during rhythmic voluntary movement of the opposite limb. J Physiol 2004; 560 (Pt 3): 929–40
Delwaide PJ, Sabatino M, Pepin JL, et al. Reinforcement of reciprocal inhibition by contralateral movements in man. Exp Neuro11988; 99: 75–98
Sabatino M, Caravaglios G, Sardo P, et al. Evidence of a contralateral motor influence on reciprocal inhibition in man. J Neural Transm Park Dis Dement Sect 1992; 4: 257–66
Sahn YR, Jung HY, Kaelin-Lang A, et al. Excitability of the ipsilateral motor cortex during phasic voluntary hand movement. Exp Brain Res 2003; 148: 176–85
Muellbacher W, Facchini S, Boroojerdi B, et al. Changes in motor cortex excitability during ipsilateral hand muscle activation in humans. Clin Neurophysiol 2000; 111 (2): 344–9
Stedman A, Davey NJ, Ellaway PH. Facilitation of human first dorsal interosseous muscle responses to transcranial magnetic stimulation during voluntary contraction of the contralateral homonymous muscle. Muscle Nerve 1998; 21 (8): 1033–9
Stinear CM, Walker KS, Byblow WD. Symmetric facilitation between motor cortices during contraction of ipsilateral hand muscles. Exp Brain Res 2001; 139: 101–5
Liepert J, Dettmers C, Terborg C, et al. Inhibition of ipsilateral motor cortex during phasic generation of low force. Clin Neurophysiol 2001; 112 (1): 114–21
Dettmers C, Fink GR, Lemon RN, et al. Relation between cerebral activity and force in the motor areas of the human brain. J NeurophysioI 1995; 74 (2): 802–15
Muellbacher W, Ziemann U, Boroojerdi B, et al. Role of the human motor cortex in rapid motor learning. Exp Brain Res2001; 136: 431–8
Muellbacher W, Ziemann U, Wissel J, et al. Early consolidation in human primary motor cortex. Nature 2002; 415 (6872):640–4
Teixeira LA, Caminha LQ. Intermanual transfer of force control is modulated by asymmetry of muscular strength. Exp BrainRes 2003; 149: 312–9
Teixeira LA. Timing and force components in bilateral transfer of learning. Brain Cogn 2000; 44 (3): 455–69
Weeks DL, Wallace SA, Anderson DI. Training with an upperlirm prosthetic simulator to enhance transfer of skill across limbs. Arch Phys Med Rehabil 2003; 84 (3): 437–43
Carroll TJ, Riek S, Carson RG. Neural adaptation to resistance training: implications for movement control. Sports Med 2001;31 (12): 829–40
Rutherford OM, Jones DA. The role of learning and coordination in strength training. Eur J Appl Physiol 1986; 55: 100–5
Hakkinen K, Alen M, Kallinen M, et al. Neuromuscular adaptation during prolonged strength training, detraining and restrength-training in middle-aged and elderly people. Eur JAppl Physiol 2000; 83: 51–62
Hess CW, Mills KR, Murray NM. Magnetic stimulation of the human brain: facilitation of motor responses by voluntary contraction of ipsilateral and contralateral muscles with additional observations on an amputee. Neurosci Lett 1986; 71 (2):235–40
Ferbert A, Priori A, Rothwell JC, et al. Interhemispheric inhibition of the human motor cortex. J Physiol 1992; 453: 525–46
Di Lazzaro V, Oliviero A, Profice P, et al. Direct demonstration of interhemispheric inhibition of the human motor cortex produced by transcranial magnetic stimulation. Exp Brain Res1999; 124: 520–4
Hanajima R, Ugawa Y, Machii K, et al. Interhemispheric facilitation of the hand motor area in humans. J Physiol 2001; 531(Pt 3): 849–59
Ugawa Y, Hanajima R, Kanazawa I. Interhemispheric facilitation of the hand area of the human motor cortex. Neurosci Lett 1993; 160 (2): 153–5
Warbrooke SA, Byblow WD. Modulation of interhemispheric inhibition during passive movement of the upper limb reflects changes in motor cortical excitability. Exp Brain Res 2004; 156 (1), 11–9
Asanuma H, Okuda O. Effects of transcallosal volleys on pyramidal tract cell activity of cat. J Neurophysiol 1962; 25:198–208
Matsunami K, Hamada I. Effects of stimulation of corpus callosum on precentral neuron activity in the awake monkey. J Neurophysiol 1984; 52 (4): 676–91
Gerloff C, Cohen LG, Floeter MK, et al. Inhibitory influence of the ipsilateral motor cortex on responses to stimulation of the human cortex and pyramidal tract. J Physiol 1998; 510 (Pt 1):249–59
Cracco RQ, Amassian VB, Maccabee PJ, et al. Comparison of human transcallosal responses evoked by magnetic coil and electrical stimulation. Electroencephalogr Clin Neurophysiol 1989; 74 (6): 417–24
Lagerquist O, Zehr EP, Docherty D. Increased spinal reflex excitability is not associated with neural plasticity underlying the cross-education effect. J Appl Physiol 2006; 100: 83–90
Hultborn H, Illert M, Santini M. Convergence on interneurones mediating the reciprocal Ia inhibition of motoneurones I: disynapticIa inhibition of Ia inhibitory interneurones. Acta Physiol Scand 1976; 96 (2): 193–201
Baldissera F, Cavallari P, Fournier E, et al. Evidence for mutual inhibition of opposite Ia interneurones in the human upper limb. Exp Brain Res 1987; 66 (1): 106–14
Lundberg A, Weight F. Signalling of reciprocal 1 a inhibition by the ventral spinocerebellar tract. Brain Res 1970; 23 (1):109–11
Hultborn H, Jankowska E, Lindstrom S, et al. Recurrent inhibition from the motor axon collaterals of transmission in the la inhibitory pathway to motoneurones. J Physiol 1971; 215:591–612
Hultborn H, Jankowska E, Lindstrom S. Relative contribution from different nerves to recurrent depression of la IPSPs inmotoneurones. J Physiol 1971; 215: 637–64
Katz R, Pierrot-Deseilligny E. Recurrent inhibition in humans. Prog Neurobiol 1998; 57 (3): 325–55
Harrison PJ, Zytnicki D. Crossed action of group 1 muscle afferents in the cat. J Physiol 1984; 356: 263–73
Delwaide PJ, Pepin JL. The influence of contralateral primary afferents on la inhibitory interneurones in humans. J Physiol 1991; 439: 161–79
Sabatino M, Sardo P, Ferraro G, et al. Bilateral reciprocal organisation in man: focus on 1A interneurone. J Neural Transm Gen Sect 1994; 96: 31–9
Jankowska E, Padel Y, Zarzecki P. Crossed disynaptic inhibition of sacral motoneurones. J Physiol 1978; 285: 425–44
Hultborn H, Pierror-Deseilligny E. Changes in recurrent inhibition during voluntary soleus contractions in man studied by an H-reflex technique. J Physiol 1979; 297: 229–51
Strens LH, Fogelson N, Shanahan P, et al. The ipsilateral human motor cortex can functionally compensate for acute contralateral motor cortex dysfunction. Curr Biol 2003; 13 (14): 1201–5
Siebner HR, Rothwell J. Transcranial magnetic stimulation: new insights into representational cortical plasticity. Exp Brain Res 2003; 148 (1): 1–16
Hallett M, Wassermann EM, Pascual-Leone A, et al. Repetitive transcranial magnetic stimulation. Electroencephalogr Clin Neurophysiol Suppl 1999; 52: 105–13
Cook TW. Studies in cross education I: mirror tracing the starshaped maze. J Exp Psychol 1933; 16: 144–60
Parlow SE, Kinsbourne M. Asymmetrical transfer of braille acquisition between hands. Brain Lang 1990; 39 (2): 319–30
Thut G, Cook ND, Regard M, et al. Intermanual transfer of proximal and distal motor engrams in humans. Exp Brain Res 1996; 108 (2): 321–7
Hicks RE, Gualtieri CT, Schroeder SR. Cognitive and motor corrponents of bilateral transfer. Am J Psychol 1983; 96:223–8
Morton SM, Lang CE, Bastian AJ. Inter- and intra-limb generalization of adaptation during catching. Exp Brain Res 2001; 141(4),43–45
Choe CS, Walsh RB. Variables affecting the intermanual transfer and decay after prism adaptation. J Exp Psychol 1974; 102:1076–84
Elliot D, Roy EA. Interlimb transfer after adaptation to visual displacement: patterns predicted from the functional closeness of limb neural control centres. Perception 1981; 10: 383–9
Sathian K, Zangaladze A. Perceptual learning in tactile hyperacuity: complete intermanual transfer but limited retention. Exp Brain Res 1998; 118 (1): 131–4
Gordon AM, Forssberg H, Iwasaki N. Formation and lateralization of internal representations underlying motor commands during precision grip. Neuropsychologia 1994; 32 (5): 555–68
Dizio P, Lackner JR. Motor adaptation to Coriolis force perturbations of reaching movements: end point but not trajectory adaptation transfers to the nonexposed arm. J Neurophysiol 1995; 74 (4): 1787–92
Farthing JP, Chilibeck PD, Binsted G. Cross-education of arm muscular strength is unidirectional in right-handed individuals. Med Sci Sports Exerc 2005; 37 (9): 1594–600
Bussey TJ, Wise SP, Murray EA. Interaction of ventral and orbital prefrontal cortex with inferotemporal cortex in conditional visuomotor learning. Behav Neurosci 2002; 116 (4):703–15
Bussey TJ, Wise SP, Murray EA. The role of ventral and orbital prefrontal cortex in conditional visuomotor learning and strategy use in rhesus monkeys (Macaca mulatta). Behav Neurosci 2001; 115 (5): 971–82
White IM, Wise SP. Rule-dependent neuronal activity in the prefrontal cortex. Exp Brain Res 1999; 126 (3): 315–35
Sanes IN. Neocortical mechanisms in motor learning. Curr Opin Neurobiol 2003; 13 (2): 225–31
Shadmehr R, Holcorm HH. Neural correlates of motor memory consolidation. Science 1997; 277 (5327): 821–5
Doyon J, Penhune V, Ungerleider LG. Distinct contribution of the cortico-striatal and cortico-cerebellar systems to motor skill learning. Neuropsychologia 2003; 41 (3): 252–62
Curtis HJ. Intracortical connections of corpus callosum as indicated by evoked potentials. J Neurophysiol 1940; 3: 407–13
Eliassen JC, Baynes K, Gazzaniga MS. Direction information coordinated via the posterior third of the corpus callosum during bimanual movements. Exp Brain Res 1999; 128 (4):573–7
Gazzaniga MS. Cerebral specialization and interhemispheric communication: does the corpus callosum enable the humancondition? Brain 2000; 123 (PI: 7): 1293–326
Rouiller EM, Babalian A, Kazennikov O, et al. Transcallosal connections of the distal forelimb representations of the primary and supplementary motor cortical areas in macaque monkeys. Exp Brain Res 1994; 102 (2): 227–43
Gould HJ, Cusick CG, Pons TP, et al. The relationship of corpus callosum connections to electrical stimulation maps of motor, supplementary motor, and the frontal eye fields in owl monkeys. J Comp Neurol 1986; 247 (3): 297–325
Carson RG. Neural pathways mediating bilateral interactions between the upperlirms. Brain Res Rev 2005; 49 (3): 641–62
Acknowledgements
This work is funded by the Australian Research Council. The authors thank Professors Simon Gandevia and Richard Carson for valuable comments on the review. The authors have no conflicts of interest that are directly relevant to the content of this review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, M., Carroll, T.J. Cross Education. Sports Med 37, 1–14 (2007). https://doi.org/10.2165/00007256-200737010-00001
Published:
Issue Date:
DOI: https://doi.org/10.2165/00007256-200737010-00001