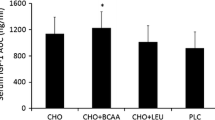
Abstract
Leucine, isoleucine and valine, the branched-chain amino acids (BCAA), make up about one-third of muscle protein. Of these, leucine has been the most thoroughly investigated because its oxidation rate is higher than that of isoleucine or valine. Leucine also stimulates protein synthesis in muscle and is closely associated with the release of gluconeogenic precursors, such as alanine, from muscle. Significant decreases in plasma or serum levels of leucine occur following aerobic (11 to 33%), anaerobic lactic (5 to 8%) and strength exercise (30%) sessions. In skeletal muscle, there is a decrease in leucine level and a reduction in glycogen stores during exhaustive aerobic exercise. Basal fasting serum leucine levels decrease by 20% during 5 weeks of speed and strength training in power-trained athletes on a daily protein intake of 1.26 g/kg bodyweight.
The leucine content of protein is assumed to vary between 5 and 10%. There are suggestions that the current recommended dietary intake of leucine be increased from 14 mg/kg bodyweight/day to a minimum of 45 mg/kg bodyweight/day for sedentary individuals, and more for those participating in intensive training in order to optimise rates of whole body protein synthesis.
Consumption of BCAA(30 to 35% leucine) before or during endurance exercise may prevent or decrease the net rate of protein degradation, may improve both mental and physical performance and may have a sparing effect on muscle glycogen degradation and depletion of muscle glycogen stores.However, leucine supplementation (200 mg/kg bodyweight) 50 minutes before anaerobic running exercise had no effect on performance. During 5 weeks of strength and speed training, leucine supplementation of 50 mg/kg bodyweight/day, supplementary to a daily protein intake of 1.26 g/kg bodyweight/day, appeared to prevent the decrease in the serum leucine levels in power-trained athletes.
According to 1 study, dietary supplementation of the leucine metabolite β-hydroxy-β-methylbutyrate (HMB) 3 g/day to humans undertaking intensive resistance training exercise resulted in an increased deposition of fat-free mass and an accompanying increase in strength.Muscle proteolysiswas also decreased with HMB, accompanied by lower plasma levels of enzymes indicating muscle damage and an average 50% decrease in plasma essential amino acid levels. Furthermore, BCAA supplementation (76% leucine) in combination with moderate energy restriction has been shown to induce significant and preferential losses of visceral adipose tissue and to allow maintenance of a high level of performance.
Caution must be paid when interpreting the limited number of studies in this area since, in many studies, leucine has been supplemented as part of a mixture of BCAA. Consequently, further research into the effects of leucine supplementation alone is needed.
Similar content being viewed by others
References
Young V. The role of skeletal and cardiac muscle in the regulation of protein metabolism. In: Munro HN, editor. Mammalian protein metabolism. New York: Academic Press, 1970: 585–611
Waterlow JC, Garlick PJ, Millward DJ. Protein turnover in mammalian tissues and in the whole body. New York: Elsevier, 1978
Meister A. Biochemistry of the amino acids. New York: Academic Press, 1965
Devlin TM. Textbook of biochemistry with clinical correlations. 2nd ed. New York: Wiley, 1986
Goldberg AL, Odessey R. Oxidation of amino acids by diaphragms from fed and fasted rats. Am J Physiol 1972; 223: 1384–91
White TP, Brooks GA. Up14-C glucose, -alanine, and -leucine oxidation in rats at rest and two intensities of running [abstract]. Am J Physiol 1981; 240: E155
Felig P, Wahren J. Amino acid metabolism in exercising man. J Clin Invest 1971; 50: 2703–14
Ahlborg G, Felig P, Hagenfeldt L, et al. Substrate turnover during prolonged exercise in man. J Clin Invest 1974; 53: 1080–90
Buse MG, Reid SS. Leucine: a possible regulator of protein turnover in muscle. J Clin Invest 1975; 56: 1250–61
Tischler ME, Desautels M, Goldberg AL. Does leucine, leucyltRNA, or some metabolite of leucine regulate protein synthesis and degradation in skeletal and cardiac muscle. J Biol Chem 1982; 257: 1613–21
Odessey R, Khairallah EA, Goldberg AL. Origin and possible significance of alanine production by skeletal muscle. J Biol Chem 1974; 249: 7623–9
Golgan M. Optimum sports nutrition. New York: Advanced Research Press, 1993
Kreider RB, Miriel V, Bertun E. Amino acid supplementation and exercise performance: analysis of the proposed ergogenic value. Sports Med 1993; 16 (3): 190–209
Nissen S, Sharp R, Ray M, et al. Effect of leucine metabolite β-hydroxy-β-methylbutyrate on muscle metabolism during resistance-exercise training. J Appl Physiol 1996; 81 (5): 2095–104
Einspahr KJ, Tharp G. Influence of endurance training on plasma amino acid concentrations in humans at rest and after intense exercise. Int J Sports Med 1989; 10: 233–6
Scriver CR, Gregory MD, Sovetts D, et al. Normal plasma free amino acid values in adults: the influence of some common physiological variables. Metabolism 1985; 9: 868–73
Mero A, Pitkänen H, Oja SS, et al. Leucine supplementation and serum amino acids, testosterone, cortisol and growth hormone in male power athletes during training. J Sports Med Phys Fitness 1997; 37 (2): 137–45
Blomstrand E, Ek S, Newsholme EA. Influence of ingesting a solution of branched-chain amino acids on plasma andmuscle concentrations of amino acids during prolonged submaximal exercise. Nutrition 1996; 12: 485–90
Bergström J, Fürst P, Hultman E. Free amino acids in muscle tissue and plasma during exercise in man. Clin Physiol 1985; 5: 155–60
Turinsky I, Long CL. Free amino acids in muscle: effect of muscle fiber population and denervation. Am J Physiol 1990; 258: E485–91
Strüder HK, Hollmann W, Duperly J, et al. Amino acid metabolism in tennis and its possible influence on the neuroendocrine system. Br J Sports Med 1995; 1: 28–30
Neumann G, Steinbach K. Veränderungen der verzweigtkettigen Aminosäuren Valin, Leucin und Isoleucin nachMarathon- und 100 km-Lauf. Med Sport Berlin 1990; 30: 249–53
Henriksson J. Effect of exercise on amino acid concentrations in skeletal muscle and plasma. J Exp Biol 1991; 160: 149–65
Wolfe RR, Goodenough RD, Wolfe MH, et al. Isotopic analysis of leucine and urea metabolism in exercising humans. J Appl Physiol 1982; 52: 458–66
Nie ZT, Henriksson J. In-vitro stimulation of the rat epitrochelaris muscle: III. Endogenous levels of branchedchain amino acids are maintained during acute contractions even in the absence of an exogenous supply. Acta Physiol Scand 1989; 137: 543–4
Mero A, Pitkänen H, Takala T, et al. Plasma amino acid responses to two various anaerobic running exercises [abstract]. Med Sci Sports Exerc 1995; 27 (5): S12
Mero A, Miikkulainen H, Riski J, et al. Effects of bovine colostrum supplementation on serum IGF-I, IgG, hormone, and saliva IgAduring training. J Appl Physiol 1997; 83 (4): 1144–51
Mero A, Pitkänen HT, Pöntinen PJ, et al. Acute serum amino acid responses to one strength training session [abstract]. Med Sci Sports Exerc 1998; 30 (5): S182
Mero A, Koistinen M, Takala T, et al. Serum amino acid and hormonal responses to training in male power athletes [abstract]. Med Sci Sports Exerc 1996; 28 (5): S25
Lemon PWR. Protein and amino acid needs of the strength athlete. Int J Sport Nutr 1991; 1: 127–45
Hood DA, Terjung RL. Amino acidmetabolismduring exercise and following endurance training. Sports Med 1990; 9 (1): 23–35
Young VR, Bier DM. A kinetic approach to the determination of human amino acid requirements. Nutr Rev 1987; 45: 289–98
Ruderman NB. Muscle amino acid metabolism and gluconeogenesis. Annu Rev Med 1975; 26: 245–58
Anderson L, Dibble MV, Turkki PR, et al., editors. Nurition in health and disease. Philadelphia (PA): JB Lippincott, 1982
US Food and Nutrition Board. Recommended Dietary Allowance (RDA). Vol. 10. Washington, DC: National Academy Press, 1989
Lemon PWR. Are dietary protein need affected by regular exercise? News on Sport Nutrition. Insider. Maastricht: Isostar Sport Nutrition Foundation 1994; 2 (3): 1–4
Hagg SA, Morse EL, Adibi SA. Effect of exercise on rates of oxidation, turnover, and plasma clearance of leucine in human subjects. Am J Physiol 1982; 242: E407–10
Lamont LS, Patel DG, Kalha SC. Leucine kinetics in endurance-trained humans. J Appl Physiol 1990; 69 (1): 1–6
Phillips SM, Atkinson SA, Tarnopolsky MA, et al. Gender differences in leucine kinetics and nitrogen balance in endurance athletes. J Appl Physiol 1993; 75 (5): 2134–41
Knapik J, Meredith C, Jones B, et al. Leucine metabolism during fasting and exercise. J Appl Physiol 1991; 70 (1): 43–7
Eriksson S, Hagenfeldt L, Wahre J. Acomparison of the effects of intravenous infusion of individual branched-chain amino acids on blood amino acid levels in man. Clin Sci 1981; 60: 95–100
Oxender DL, Christensen HN. Distinct mediating systems for the transport of neutral amino acids by the Ehrlich cell. J Biol Chem 1963; 238: 3686–99
Winter CG, Christensen HN. Migration of amino acids across the membrane of the human erythrocyte. J Biol Chem 1964; 239: 872–8
Alvestrand A, Hagenfeldt L, Merli M, et al. Influence of leucine infusion on intracellular amino acids in humans. Eur J Clin Invest 1990; 20: 293–8
Blomstrand E, Newsholme EA. Effect of branched-chain amino acid supplementation on the exercise-induced change in aromatic amino acid concentration in human muscle. Acta Physiol Scand 1992; 146: 293–8
Blomstrand E, Hassmén P, Ekblom B, et al. Administration of branched-chain amino acids during sustained exercise: effects on performance and on plasma concentration of some amino acids. Eur J Appl Physiol 1991; 63: 83–8
Varnier M, Sarto P, Martines D, et al. Effect of infusing branched-chain amino acid during incremental exercise with reduced muscle glycogen content. Eur J Appl Physiol 1994; 69: 26–31
Mero A, Nummela A, Rusko H, et al. Influence of leucine supplementation on serum amino acid concentration and anaerobic running performance [abstract]. Med Sci Sports Exerc 1997; 29 (5): S192
Vukovich MD, Sharp RL, Kesl LD, et al. Effects of a low-dose amino acid supplement on adaptations to cycling training in untrained individuals. Int J Sport Nutr 1997; 7: 298–309
Sahlin K, Henriksson J. Buffer capacity and lactate accumulation in skeletal muscle of trained and untrained men. Acta Physiol Scand 1984; 122: 331–9
Hutson SM, Zapalowski C, Cree TC, et al. Regulation of leucine and alpha-ketoisocaproic acid metabolism in skeletal muscle. J Biol Chem 1980; 255: 2418–26
Nissen SL, Haymond MW. Effects of fasting on flux and interconversion of leucine and alpha-ketoisocaproate in vivo. Am J Physiol 1981; 241: E67–71
Odessey R, Goldberg AL. Oxidation of leucine by rat skeletal muscle. Am J Physiol 1972; 223: 1376–83
Fielding RA, Evans WJ, Hughes VA, et al. The effects of high intensity exercise on muscle and plasma levels of alphaketoisocaproic acid. Eur J Appl Physiol 1986; 55: 482–5
Van Koevering M, Nissen S. Oxidation of leucine and α- ketoisocaproate to β- hydroxy-β-methylbutyrate in vivo. Am J Physiol 1992; 262: E27–31
Mourier A, Bigard AX, de Kerviler E, et al. Combined effects of caloric restriction and branched-chain amino acid supplementation on body composition and exercise performance in elite wrestlers. Int J Sports Med 1997; 18: 47–55
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mero, A. Leucine Supplementation and Intensive Training. Sports Med 27, 347–358 (1999). https://doi.org/10.2165/00007256-199927060-00001
Published:
Issue Date:
DOI: https://doi.org/10.2165/00007256-199927060-00001