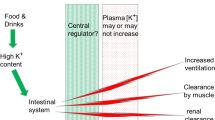
Summary
Muscular fatigue is manifested by a decline in force- or power-generating capacity and may be prominent in both submaximal and maximal contractions. Disturbances in muscle electrolytes play an important role in the development of muscular fatigue. Intense muscular contraction is accompanied by an increased muscle water content, distributed in both intracellular and extra-cellular spaces. This water influx will modify ionic changes in both compartments. Changes in muscle intracellular electrolyte concentrations with intense contraction may be summarised as including decreases in potassium (6 to 20%) and in creatine phosphate (up to 70 to 100%) and increases in lactate (more than 10-fold), sodium (2-fold) and small, variable increases in chloride. The net result of these intracellular ionic concentration changes with exercise will be a reduction in the intracellular strong ion difference, with a consequent marked rise in intracellular hydrogen ion concentration. This intracellular acidosis has been linked with fatigue via impairment of regulatory and contractile protein function, calcium regulation and metabolism. Potassium efflux from the contracting muscle cell dramatically decreases the intracellular to extracellular potassium ratio, leading to depolarisation of sarcolemmal and t-tubular membranes. Surprisingly little research has investigated the effects of intense exercise training on electrolyte regulation and fatigue. Intense sprint training in man attenuates muscular fatigue during short term maximal exercise. This is accompanied by improved potassium homeostasis and possibly, improved regulation of muscular acidosis, both factors which may reduce muscular fatigue.
Similar content being viewed by others
References
Adrian RH. The effect of internal and external potassium concentrations on the membrane potential of frog muscle. Journal of Physiology 133: 631–658, 1956
Adrian RH, Peachey LD. Reconstruction of the action potential of frog sartorius muscle. Journal of Physiology 235: 103–131, 1973
Ahlborg B, Bergstrom J, Ekelund L, Hultman E. Muscle glycogen and muscle electrolytes during prolonged physical exercise. Acta Physiologica Scandinavica 70: 129–142, 1967
Aickin CC, Thomas RC. An investigation of the ionic mechanism of intracellular pH regulation in mouse soleus muscle fibres. Journal of Physiology 273: 295–316, 1977
Allen DG, Lee JA, Westerblad H. Intracellular calcium and tension during fatigue in isolated single muscle fibres from Xenopus Laevis. Journal of Physiology 415: 433–458, 1989
Ashley GC, Ridgway EB. On the relationships between membrane potential, calcium transient and tension in single barnacle muscle fibres. Journal of Physiology 209: 105–130, 1990
Bang O. The lactate content of the blood during and after muscular exercise in man. Skandinavian Archives of Physiology (Suppl. 10): 51–81, 1936
Bellemare F, Garzaniti N. Failure of neuromuscular propagation during human maximal voluntary contraction. Journal of Applied Physiology 64, 1084–1093, 1988
Bergstrom J, Guarniera G, Hultman E. Carbohydrate metabolism and electrolyte changes in human muscle tissue during heavy work. Journal of Applied Physiology 30: 122–125, 1971
Bigland-Ritchie B. EMG and fatigue of human voluntary and stimulated contractions. In Human muscle fatigue: physiological mechanisms, pp. 130–156, Pitman Medical, London (Ciba Foundation symposium 82), 1981
Byrd SK, Bode AK, Klug GA. Effects of exercise of varying duration on sarcoplasmic reticulum function. Journal of Applied Physiology 66: 1383–1389, 1989a
Byrd SK, McCutcheon LJ, Hodgson DR, Gollnick PD. Altered sarcoplasmic reticulum function after high intensity exercise. Journal of Applied Physiology 67: 2072–2077, 1989b
Castle NA, Haylett DG. Effect of channel blockers on potassium efflux from metabolically exhausted frog skeletal muscle. Jour nal of Physiology 383: 31–43, 1987
Chasiotis D, Hultman E, Sahlin K. Acidotic depression of cyclic AMP accumulation and phosphorylase b to a transformation in skeletal muscle of man. Journal of Physiology 335: 197–204, 1982
Cheetham ME, Boobis LH, Brooks S, Williams C. Human muscle metabolism during sprint running. Journal of Applied Physiology 61: 54–60, 1986
Christy RK. The migrations of chlorine ions. Journal of Physiology 63: X, 1927
Clausen T. Regulation of active Na+−K+ transport in skeletal muscle. Physiological Reviews 66: 542–580, 1986
Cooke R, Franks K, Luciani GB, Pate E. The inhibition of rabbit skeletal muscle contraction by hydrogen ions and phosphate. Journal of Physiology 395: 77–97, 1988
Costill DL, Saltin B. Muscle glycogen and electrolytes following exercise and thermal dehydration. In Howald H & Poortmans JR (Eds) Metabolic adaptation to prolonged physical exercise, Biochemistry of exercise II, pp. 352–360. Birkhauser Verlag, Basel, 1975
Davies NW. Modulation of ATP-sensitive K+ channels in skeletal muscle by intracellular protons. Nature 343: 375–377, 1990
Dill DB, Talbot JH, Edwards HT. Studies in muscular activity. VI. Response of several individuals to a fixed task. Journal of Physiology 69: 268–305, 1930
Donaldson SKB, Hermansen L. Differential, direct effects of H+ on Ca2+-activated force of skinned fibres from the soleus, cardiac and adductor magnus muscles of rabbits. Pfliigers Archiv 376: 55–65, 1978
Edwards RHT. Human muscle function and fatigue. In Human muscle fatigue: physiological mechanisms, pp. 1–18, Pitman Medical, London (Ciba Foundation Symposium 82), 1981
Fabiato A, Fabiato F. Effects of pH on the myofilaments and the sarcoplasmic reticulum of skinned cells from cardiac and skeletal muscles. Journal of Physiology 276, 233–255, 1978
Fenn WO. Electrolytes in muscle. Physiological Reviews 16: 450–487, 1936
Fenn WO, Cobb DM. Electrolyte changes in muscle during activity. American Journal of Physiology 115: 345–356, 1936
Fink R, Luttgau HC. An evaluation of the membrane constants and the potassium conductance in metabolically exhausted muscle fibres. Journal of Physiology 263: 215–238, 1976
Gollnick PD, Korge P, Karpakka J, Saltin B. Elongation of skeletal muscle relaxation during exercise is linked to reduced calcium uptake by the sarcoplasmic reticulum in man. Acta Physiologica Scandinavica 142: 135–136, 1991
Hermansen L, Osnes J. Blood and muscle pH after maximal exercise in man. Journal of Applied Physiology 32: 304–308, 1972
Hermansen L, Orheim A, Sejersted OM. Metabolic acidosis and changes in water and electrolyte balance in relation to fatigue during maximal exercise of short duration. International Journal of Sports Medicine 5: 110–115, 1984
Hill AV, Lupton H. Muscular exercise, lactic acid, and the supply and utilization of oxygen. Quarterly Journal of Medicine, 135–171, 1923
Hirche H, Schumacher & Hagemann H. Extracellular K+ concentration and K+ balance of the gastrocnemius muscle of the dog during exercise. Pflügers Archiv 387: 231–237, 1980
Hnik P, Vyskocil F, Ujec E, Vojsada R, Rehfeldt H. Work-induced potassium loss from skeletal muscles and its physiological implications. Biochemistry of Exercise. VI. International Series Sport Science 16: 345–364, 1976
Hodgkin AL, Horowitcz P. Movements of Na and K in single muscle fibres. Journal of Physiology 145: 405–432, 1959a
Hodgkin AL, Horowitcz P. The influence of potassium on single muscle fibres. Journal of Physiology 148: 127–160, 1959b
Hodgkin AL, Huxley AF. Current carried by sodium and potassium ions through the membrane of the giant axon of Loligo. Journal of Physiology 116: 449–472, 1952a
Hodgkin AL, Huxley AF. The dual effect of membrane potential on sodium conductance in the giant axon of Loligo. Journal of Physiology 116: 497–506, 1952b
Hodgkin AL, Katz B. The effect of sodium ions on the electrical activity of the giant axon of the squid. Journal of Physiology 108: 37–77, 1949
Jones DA. Muscle fatigue due to changes beyond the neuromuscular junction. In Human muscle fatigue: physiological mechanisms, pp. 178–196, Pitman Medical, London (Ciba Foundation Symposium 82), 1981
Jones DA, Bigland-Ritchie B. Electrical and contractile changes in muscle fatigue. Biochemistry of exercise. VI. International Series Sport Science 16: 377–392, 1986
Jones NL, [H+] Control in exercise: concepts and controversies. Biochemistry of exercise. VII. International Series Sport Science 21: 333–340, 1990
Juel C. Potassium and sodium shifts during in vitro isometric muscle contraction, and the time course of the ion-gradient recovery. Pflügers Archiv 406: 458–463, 1986
Juel C. The effect of beta2-adrenoceptor activation on ion-shifts and fatigue in mouse soleus muscles stimulated in vitro. Acta Physiologica Scandinavica 134: 209–216, 1988
Katz A, Sahlin K, Juhlin-Dannfelt A. Effect of beta-adrenoceptor blockade on H+ and K+ flux in exercising humans. Journal of Applied Physiology 59: 336–341, 1985
Keys A. Exchanges between blood plasma and tissue fluid in man. Science 85: 317–318, 1937
Kowalchuk JM, Heigenhauser GJF, Lindinger MI, Sutton JR, Jones NL. Factors influencing hydrogen ion concentration in muscle after intense exercise. Journal of Applied Physiology 65: 2080–2089, 1988
Lannergren J, Westerblad H. Maximum tension and force-velocity properties of fatigued single Xenopus muscle fibres studies by caffeine and high K+. Journal of Physiology 409: 473–490, 1989
Laurell H, Pernow B. Effect of exercise on plasma potassium in man. Acta Physiologica Scandinavica 66: 241–242, 1966
Lindinger MI, Heigenhauser GJF. Intracellular ion content of skeletal muscle measured by instrumental neutron activation analysis. Journal of Applied Physiology 63: 426–433, 1987
Lindinger MI, Heigenhauser GJF. Ion fluxes during tetanic stimulation in isolated perfused rat hindlimb. American Journal of Physiology 254: R117–R126, 1988
Lindinger MI, Heigenhauser GJF. Acid-base systems in skeletal muscle and their response to exercise. Biochemistry of exercise. VII. International Series Sport Science 21: 341–358, 1990
Lindinger MI, Heigenhauser GJF. The roles of ion fluxes in skeletal muscle fatigue. Canadian Journal of Physiology and Pharmacology 69: 246–253, 1991
Lindinger MI, Heigenhauser GJF, Spriet LL. Effects of intense swimming and tetanic electrical stimulation on skeletal muscle ions and metabolites. Journal of Applied Physiology 63: 2331–2339, 1987
Lindinger MI, Sjogaard G. Potassium regulation during exercise and recovery. Sports Medicine 11: 382–401, 1991
Lynch GS, McKenna MJ, Williams DA. Sprint-training and the effect of lowered pH on some contractile properties of human skeletal muscle fibres. Proceedings of the Australian Physiology and Pharmacology Society 20: 165P, 1989
McKenna MJ. The effects of sprint training on electrolyte, acid-base regulation and fatigue during intense exercise in man. Ph.D. Thesis, University of Melbourne, Victoria, 1991
McKenna MJ, Heigenhauser GJF, McKelvie RS, Sutton JR, MacDougall JD, Jones NL. The effects of sprint training upon changes in plasma electrolyte concentrations across active muscle during intense exercise. Proceedings of the Australian Physiology and Pharmacology Society 20: 120P, 1989
McKenna MJ, Schmidt TA, Hargreaves M, Cameron L, Skinner SL, Kjeldsen K. Sprint training improves potassium regulation and reduces muscular fatigue during intense exercise. Proceedings of the Australian Physiology and Pharmacology Society 21: 138P, 1990
Medbø JI, Sejersted OM. Acid-base and electrolyte balance after exhausting exercise in endurance trained and sprint-trained subjects. Acta Physiologica Scandinavica 125: 97–109, 1985
Medbø JI, Sejersted OM. Plasma potassium changes with high intensity exercise. Journal of Physiology 421: 105–122, 1990
Merton PA, Hill DK, Morton HB. Indirect and direct stimulation of fatigued human muscle. In Human muscle fatigue: physiological mechanisms, pp 120–129, Pitman Medical, London (Ciba Foundation Symposium 82), 1981
Metzger JM, Moss RL. Greater hydrogen ion induced depression of tension and velocity in skinned single fibres of rat fast than slow muscles. Journal of Physiology 393, 727–742, 1987
Milner-Brown HS, Miller RG. Muscle membrane excitation and impulse propagation velocity are reduced during muscle fatigue. Muscle and Nerve 9: 367–374, 1986
Nevill ME, Boobis LH, Brooks S, Williams C. Effect of training on muscle metabolism during treadmill sprinting. Journal of Applied Physiology 67: 2376–2382, 1989
Nosek TM, Fender KY, Godt RE. It is diprotonated inorganic phosphate that depresses force in skinned skeletal muscle fibres. Science 236: 191–193, 1987
Owles WH. Alterations in the lactic acid content of the blood as a result of light exercise, and associated changes in the CO2 combining power of the blood and in the alveolar CO2 pressure. Journal of Physiology 69: 214–237, 1930
Pallotta BS, Magleby KL, Barrett JN. Single channel recordings of Ca2+-activated K+ currents in rat muscle cell culture. Nature 293: 471–474, 1981
Rolett EL, Strange S, Sjogaard G, Kiens B, Saltin B. Beta2-Adrenergic stimulation does not prevent potassium loss from exercising quadriceps muscle. American Journal of Physiology 258: R1192–R1200, 1990
Ryffel JH. Experiments in lactic acid formation in man. Journal of Physiology 39: 29P–32P, 1909
Sahlin K, Alverstrand A, Brandt R, Hultman E. Intracellular pH and bicarbonate concentration in human muscle during recovery from exercise. Journal of Applied Physiology 45: 474–480, 1978
Saltin B, Sjogaard G, Strange S, Juel C. Redistribution of potassium in the human body during muscular exercise; its role to maintain whole body homeostasis. In Shiraki K, Yousef MK (Eds) Man in stressful environments; thermal and work physiology, Charles C Thomas, Springfield, 111., 1987
Sejersted OM, Hallen J. Na, K homeostasis of skeletal muscle during activation. Medicine and Sport Science 26: pp. 1–11, Karger, Basel, 1987
Sejerted OM, Vollestad NK, Medbo JI. Muscle fluid and electrolyte balance during and following exercise. Acta Physiologica Scandinavica 128 (Suppl. 556): 119–127, 1986
Sembrowich WL, Johnson D, Wang E, Hutchinson TE. Electron microprobe analysis of fatigued fast- and slow-twitch mucle. In Knuttgen et al. (Eds) Biochemistry of Exercise, International Series on Sport Science, Vol. 13, pp. 571–576, Human Kinetics Publishers, Champaign, 111. 1983
Sharp RL, Costill DL, Fink WJ, King DS. Effects of eight weeks of bicycle ergometer sprint training on human muscle buffer capacity. International Journal of Sports Medicine 7: 13–17, 1986
Sjøgaard G. Electrolytes in slow and fast muscle fibres of humans at rest and with dynamic exercise. American Journal of Physiology 245: R25–R31, 1983
Sjøgaard G. Water and electrolyte fluxes during exercise and their relation to muscle fatigue. Acta Physiologica Scandinavica 128 (Suppl. 556): 129–136, 1986
Sjøgaard G. Exercise-induced muscle fatigue; the significance of potassium. Acta Physiologica Scandinavica 140 (Suppl. 593), 1–63, 1990
Sjøgaard G. Role of exercise-induced potassium fluxes underlying muscle fatigue: a brief review. Canadian Journal of Physiology and Pharmacology 69: 238–245, 1991
Sjøgaard G, Adams RP, Saltin B. Water and ion shifts in skeletal muscle of humans with intense dynamic knee extension. American Journal of Physiology 248: R190–R196, 1985
Sjøgaard G, Saltin B. Extra- and intracellular water spaces in muscles of man at rest and with dynamic exercise. American Journal of Physiology 243: R271–R280, 1982
Skinner SL. A cause of erroneous potassium concentrations. Lancet 2: 478–480, 1961
Spriet LL, Lindinger MI, McKelvie RS, Heigenhauser GJF, Jones NL. Muscle glycogenosis and H+ concentration during maximal intermittent cycling. Journal of Applied Physiology 66: 8–13, 1989
Spruce AE, Standen NB, Stanfield PR. Voltage-dependent ATP-sensitive potassium channels of skeletal muscle membrane. Nature 316: 736–738, 1985
Sreter FA. Cell water, sodium and potassium in red and white mammalian muscles. American Journal of Physiology 205: 1295–1298, 1963
Stewart CA, Bretag AH. Membrane conductance of metabolically exhausted mammalian muscle. Proceedings of the Australian Physiology and Pharmacology Society 21: 28P, 1991
Stewart PA. How to understand acid-base: a quantitative acid-base primer for biology and medicine, Elsevier North Holland, New York, 1981
Stewart PA. Modern quantitative acid-base chemistry. Canadian Journal of Physiology and Pharmacology 61: 1444–1461, 1983
Sutton JR, Jones NL, Toews CJ. Effect of pH on muscle glycolysis during exercise. Clinical Science 61: 331–338, 1981
van Beaumont W, Strand JC, Petrovsky JS, Hipskind SG, Greenleaf JE. Changes in total plasma content of electrolytes and proteins with maximal exercise. Journal of Applied Physiology 34: 102–106, 1973
Vollestad N, Sejersted OM. Biochemical correlates of fatigue: a brief review. European Journal of Applied Physiology 57: 336–347, 1988
Vyskocil F, Hnik P, Vejsada R, Ujec E. The measurement of K+ e concentration changes in human muscles during volitional contractions. Pflugers Archiv 399: 235–237, 1983
Westerblad H, Lannergren J. Force and membrane potential during and after fatiguing, intermittent stimulation of single Xenopus muscle fibres. Acta Physiologica Scandinavica 128: 369–378, 1986
Westerblad H, Lee JA, Lamb AG, Bolsover SR, Allen DG. Spatial gradients of intracellular calcium in skeletal muscle during fatigue. Pflugers Archiv 415: 734–740, 1990
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
McKenna, M.J. The Roles of Ionic Processes in Muscular Fatigue During Intense Exercise. Sports Medicine 13, 134–145 (1992). https://doi.org/10.2165/00007256-199213020-00009
Published:
Issue Date:
DOI: https://doi.org/10.2165/00007256-199213020-00009