Abstract
Non-small cell lung cancer (NSCLC) creates a large economic and disease burden worldwide. In an era of evidence-based medicine and increasing cost pressures, it is important to understand the relative clinical and economic impact of the many drug treatment strategies available for NSCLC.
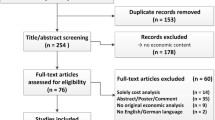
A systematic review of the peer-reviewed literature for pharmacoeconomic evaluations in the primary treatment of NSCLC published over the past decade (1 June 1997 to 1 June 2007) was conducted using the PubMed, EMBASE, BIOSIS Previews®, Harvard Review of Economic Analyses, National Institute for Health and Clinical Excellence and Canadian Agency for Drugs and Technologies in Health databases. A total of 19 studies met the inclusion/exclusion criteria. Of these studies, 58% were cost-effectiveness studies, 37% were cost-minimization studies and 5% were cost-utility studies. Most were from the EU (63%), were from the payer perspective (89%), were in advanced (stage IIIB/IV) NSCLC (84%) and were funded by drug manufacturers (68%).
Drug treatments generally were found to be cost effective compared with best supportive care. In addition, cisplatin alone or in combination appeared to provide better value than carboplatin alone or in combination. We did not identify any studies of recently approved therapeutics (e.g. erlotinib or bevacizumab). The quality of studies varied but the majority did not meet recommended guidelines for economic evaluations, with only 43% using direct comparisons, 5% of studies being cost-utility studies and 26% using either statistical analysis of patient-level data or probabilistic sensitivity analyses.
In conclusion, there are a multitude of studies examining drug treatment for NSCLC; however, few of these utilized methodological approaches consistent with recommended guidelines. Despite these limitations, it appears that drug therapy compared with no treatment provides reasonable value for money, but carrying out more detailed comparisons of various agents is challenging. Given the absence of studies on newer therapeutics and the lack of cost-utility studies, additional studies are warranted.



Similar content being viewed by others
References
World Health Organization. Revised global burden of disease (GBD) 2002 estimates. Available from URL: http:// www.who.int/healthinfo/bodgbd2002revised/en/index.html [Accessed 2007 May 9]
Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin 2005; 55(2): 74–108
American Cancer Society. Cancer facts and figures 2007. Atlanta (GA): American Cancer Society, 2007
Kutikova L, Bowman L, Chang S, et al. The economic burden of lung cancer and the associated costs of treatment failure in the United States. Lung Cancer 2005; 50(2): 143–54
The National Institute for Health and Clinical Excellence. ERG report: erlotinib for the treatment of relapsed non-small cell lung cancer. Liverpool: The National Institute for Health and Clinical Excellence, 2006
National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: non-small cell lung cancer V.2.2006 [online]. Available from URL: http://www.nccn.org/ professionals/physician_gls/PDF/nscl.pdf [Accessed 2008 Mar 19]
Ramsey SD, Howlader N, Etzioni RD, et al. Chemotherapy use, outcomes, and costs for older persons with advanced non-small-cell lung cancer: evidence from surveillance, epidemiology and end results — Medicare. J Clin Oncol 2004; 22(24): 4971–8
Mather D, Sullivan SD, Parasuraman TV. Beyond survival: economic analyses of chemotherapy in advanced, inoperable NSCLC. Oncology (Williston Park) 1998; 12(2): 199–209; discussion 210, 215–6
Drummond MF, O’Brien B, Stoddart GL, et al. Methods for the economic evaluation of health care programmes. 2nd ed. Oxford: Oxford University Press, 1997
Chiou CF, Hay JW, Wallace JF, et al. Development and validation of a grading system for the quality of cost-effectiveness studies. Medical Care 2003; 41(1): 32–44
Ng R, Hasan B, Mittmann N, et al. Economic analysis of NCIC CTG JBR.10: a randomized trial of adjuvant vinorelbine plus cisplatin compared with observation in early stage non-small-cell lung cancer. A report of the Working Group on Economic Analysis, and the Lung Disease Site Group, National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 2007; 25(16): 2256–61
Dooms CA, Lievens YN, Vansteenkiste JF. Cost-utility analysis of chemotherapy in symptomatic advanced nonsmall cell lung cancer. Eur Respir J 2006; 27(5): 895–901
Pimentel FL, Bhalla S, Laranjeira L, et al. Cost-minimization analysis for Portugal of five doublet chemotherapy regimens from two phase III trials in the treatment of advanced non-small cell lung cancer. Lung Cancer 2006; 52(3): 365–71
Neymark N, Lianes P, Smit EF, et al. Economic evaluation of three two-drug chemotherapy regimens in advanced non-small-cell lung cancer. Pharmacoeconomics 2005; 23(11): 1155–66
Novello S, Kielhorn A, Stynes G, et al. Cost-minimisation analysis comparing gemcitabine/cisplatin, paclitaxel/carboplatin and vinorelbine/cisplatin in the treatment of advanced non-small cell lung cancer in Italy. Lung Cancer 2005; 48(3): 379–87
Holmes J, Dunlop D, Hemmett L, et al. A cost-effectiveness analysis of docetaxel in the second-line treatment of non-small cell lung cancer. Pharmacoeconomics 2004; 22(9): 581–9
Billingham LJ, Bathers S, Burton A, et al. Patterns, costs and cost-effectiveness of care in a trial of chemotherapy for advanced non-small cell lung cancer. Lung Cancer 2002; 37(2): 219–25
Clegg A, Scott DA, Hewitson P, et al. Clinical and cost effectiveness of paclitaxel, docetaxel, gemcitabine, and vinorelbine in non-small cell lung cancer: a systematic review. Thorax 2002; 57(1): 20–8
Lees M, Aristides M, Maniadakis N, et al. Economic evaluation of gemcitabine alone and in combination with cisplatin in the treatment of nonsmall cell lung cancer. Pharmacoeconomics 2002; 20(5): 325–37
Leighl NB, Shepherd FA, Kwong R, et al. Economic analysis of the TAX 317 trial: docetaxel versus best supportive care as second-line therapy of advanced non-small-cell lung cancer. J Clin Oncol 2002; 20(5): 1344–52
Ramsey SD, Moinpour CM, Lovato LC, et al. Economic analysis of vinorelbine plus cisplatin versus paclitaxel plus carboplatin for advanced non-small-cell lung cancer. J Natl Cancer Inst 2002; 94(4): 291–7
Rubio-Terres C, Tisaire JL, Kobina S, et al. Cost-minimisation analysis of three regimens of chemotherapy (docetaxel-cisplatin, paclitaxel-cisplatin, paclitaxel-carboplatin) for advanced non-small-cell lung cancer. Lung Cancer 2002; 35(1): 81–9
Annemans L, Giaccone G, Vergnenegre A. The cost-effectiveness of paclitaxel (Taxol) + cisplatin is similar to that of teniposide + cisplatin in advanced non-small cell lung cancer: a multicountry analysis. Anticancer Drugs 1999; 10(6): 605–15
Khan ZM, Rascati KL, Koeller JM. Economic analysis of carboplatin versus cisplatin in lung and ovarian cancer. Pharmacoeconomics 1999; 16(1): 43–57
Sacristan JA, Kennedy-Martin T, Rosell R, et al. Economic evaluation in a randomized phase III clinical trial comparing gemcitabine/cisplatin and etoposide/cisplatin in non-small cell lung cancer. Lung Cancer 2000; 28(2): 97–107
Earle CC, Evans WK. Cost-effectiveness of paclitaxel plus cisplatin in advanced non-small-cell lung cancer. Br J Cancer 1999; 80(5–6): 815–20
Evans WK. Cost-effectiveness of vinorelbine alone or vinorelbine plus cisplatin for stage IV NSCLC. Oncology (Williston Park) 1998; 12 (3 Suppl. 4): 18–25; discussion 25-6
Tennvall GR, Fernberg JO. Economic evaluation of gemcitabine single agent therapy compared with standard treatment in stage IIIB and IV non-small cell lung cancer. Med Oncol 1998; 15(2): 129–36
Evans WK, Will BP, Berthelot JM, et al. Cost of combined modality interventions for stage III non-small-cell lung cancer. J Clin Oncol 1997; 15(9): 3038–48
Owens DK. Interpretation of cost-effectiveness analyses. J Gen Intern Med 1998; 13(10): 716–7
Shepherd FA, Dancey J, Ramlau R, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol 2000; 18(10): 2095–103
Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 2005; 353(2): 123–32
Hotta K, Matsuo K, Ueoka H, et al. Meta-analysis of randomized clinical trials comparing cisplatin to carboplatin in patients with advanced non-small-cell lung cancer. J Clin Oncol 2004; 22(19): 3852–9
Ardizzoni A, Boni L, Tiseo M, et al. Cisplatin-versus carboplatin-based chemotherapy in first-line treatment of advanced non-small-cell lung cancer: an individual patient data metaanalysis. J Natl Cancer Inst 2007; 99(11): 847–57
Gold M, Seigel J, Russell L, et al. Cost-effectiveness in health and medicine. Oxford: Oxford University Press, 1996
Acknowledgements
No sources of funding were used to assist in the preparation of this review. The authors report the following conflicts of interest: Josh Carlson has served as a consultant to Genentech, Inc. and F. Hoffmann-La Roche, Ltd. David Veenstra has received research funding and served as a consultant to Genentech, Inc., F. Hoffmann-La Roche and Bristol-Myers Squibb. Scott Ramsey has served as a consultant to Genentech, Inc. and has received research funding from Bristol-Myers Squibb.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Carlson, J.J., Veenstra, D.L. & Ramsey, S.D. Pharmacoeconomic Evaluations in the Treatment of Non-Small Cell Lung Cancer. Drugs 68, 1105–1113 (2008). https://doi.org/10.2165/00003495-200868080-00007
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-200868080-00007